Polyladderene transformed to polyacetylene using mechanochemistry
Researchers have designed a polymer that can be ‘unzipped’ from a non-conjugated, insulating form to a conjugated, conductive material by simply applying force.
Mechanical force is rarely used by researchers to trigger chemical reactions. But in the last decade polymer mechanochemistry has gained a greater level of interest, from chemists such as Yan Xia at Stanford University in the US.
Xia’s dream was to create a polymer that could completely change its properties with a simple pull. He was struggling until he spoke with a Stanford colleague, Noah Burns, who was working on the total synthesis of an exotic natural molecule called a ladderane.
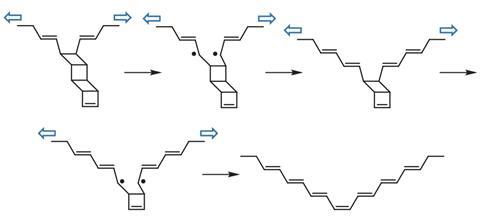
Ladderanes contain several four-membered rings fused together. ‘That ladder structure struck me as a perfect candidate for the mechanochemical transformation we have envisioned,’ says Xia. Taking this, the team designed a monomer, ladderene, which they could link to form a long polymer.
The researchers found that they could ‘unzip’ their ladderene polymer by sonicating the molecule in solution. ‘Experimentally we use sonication to “pull” this molecule,’ explains Zhixing Chen, from Xia’s group. After just 20 seconds of sonication the previously colourless solution turned blue, as the polyladderene transformed to polyacetylene. On further sonication they saw long polyacetylene nanowires form, and the reaction mixture became a dark blue.
The polymer’s mechanical metamorphosis from a non-conjugated insulating form, to a conjugated, semiconductive form results from the unzipping of many ladderene units. ‘When they break they generate a diradical transition state and then they rearrange to conjugated olefins,’ says Xia.
Tomislav Friscic, who researches mechanochemistry at McGill University in Canada, says the work is ‘an elegant use of both mechanochemistry and a non-conventional building block’.
Stephen Craig, a polymer mechanochemistry expert at Duke University in the US, agrees: ‘The thing I like most here is that the mechanically induced transformation does two things simultaneously: it dramatically changes the properties of the polymer, and it also releases an enormous amount of stored length. Both behaviours might be useful as the potential of mechanophore-based polymers continues to be developed.’
While this study was on the molecular level, the team’s goal is to create a piece of plastic on the macroscopic scale that can be pulled and turned into a metal-like material. They also want to find out ‘how the mechanical force is transduced along a very long polymer or polymer network’, says Xia. ‘That’s still a very difficult question to answer.’ While this particular transformation is one-way, the team also hopes to create materials that could transform reversibly.
References
Z Chen et al, Science, 2017, 357, 475 (DOI: 10.1126/science.aan2797)








No comments yet