Scientists in Japan have used mechanochemistry to prepare organomanganese reagents. The process avoids issues associated with solution-based preparation methods, which typically require harsh, complex or costly conditions.
Organic chemists use organometallic reagents such as organomanganese for a wide variety of synthetic transformations. Despite organomanganese reagents having beneficial properties such as low toxicity, low cost and mild reactivity, other challenges limit their use. Only highly reactive organic allylic halides or α-haloesters can react with commercial manganese metal through direct synthesis. The oxide layer covering manganese metal reduces its reactivity, and preparing arylmanganese reagents requires an activated version, Rieke manganese, which is made using strong reducing reagents like alkali metals. Alternative direct arylmanganese synthesis methods require metal additives, organic solvents and inert atmospheres, all of which pose synthetic challenges.
‘Our group is interested in the use of ball-milling methods to address outstanding challenges in conventional solution-based organic synthesis,’ explains Koji Kubota at Hokkaido University. Kubota’s team had previously used ball milling to activate bulk metals with low reactivity such as calcium. Other groups have used ball milling to activate manganese for use in organic reactions, but the mechanochemical direct synthesis of organomanganese nucleophiles had not yet been reported.
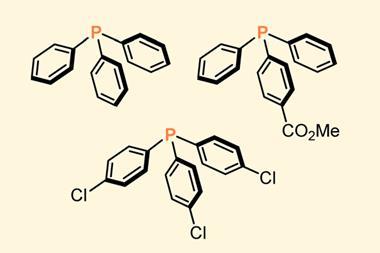
Attempting to further expand their mechanochemical activation method to prepare arylmanganese species, the team combined commercial manganese metal, aryl iodides and two equivalents of tetrahydrofuran in a stainless steel mixer mill for 90 minutes, notably, under atmospheric conditions. They initially found that aryl iodides with electron-donating and electron-withdrawing groups were unreactive, and only ortho-halogenated aryl iodides would react, while including ether additives improved the yields.
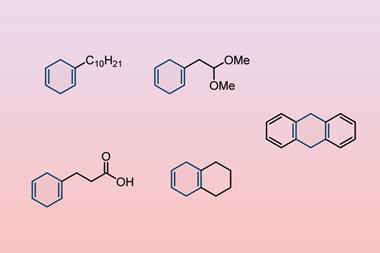
The team explored the scope of organic halides that could form organomanganese nucleophiles, reporting the first alkylmanganese reagents to be generated directly from unactivated alkyl iodide with unactivated manganese metal. ‘It’s very difficult for this reaction to proceed under conventional solution-based conditions, but this mechanochemical method opens practical applications of organomanganese chemistry in organic synthesis,’ says Kubota.
They demonstrated the applications of the arylmanganese reagents by reacting them with different electrophiles under ball milling conditions in cross-coupling and addition reactions. ‘This research has made it much easier for synthetic chemists to generate organomanganese reagents from commercially available materials,’ adds Kubota. The team plan to extend the technique to other bulk metals in the future.
The method ‘succeeds because it offers a new way to easily employ a relatively neglected metal, manganese, in organic synthesis,’ comments Timothy Hanusa, an expert in organometallic and mechanochemistry at Vanderbilt University in the US. ‘The earth-abundance of manganese is complemented by the sustainable nature of mechanochemistry, which avoids the use of potentially toxic solvents. The ability to work (briefly) under air adds some extra experimental ease to the process,’ he adds. ‘These new developments can be expected to impact research in a relatively neglected area of organic synthesis.’












No comments yet