A hybrid organic–inorganic crystal shows potent cooling properties under the application of electric fields, scientists in China report. This new type of electrocaloric material could mark an important step in developing green and efficient refrigeration.
Currently, much domestic and commercial refrigeration is delivered through the vapour-compression cycle, in which a coolant fluid is repeatedly compressed and expanded. During the expansion phase, the fluid evaporates, and energy is required to break the attraction between molecules. This energy comes from the molecules’ thermal motion, resulting in a temperature drop.
But these coolant fluids have long posed hazards to the environment, and the hunt for green alternatives is on. This involves finding another reversible process, akin to evaporation and condensation, through which thermal energy can be absorbed and released. The mechanocaloric effect, for example, relies on deformations of a solid, whereas in magnetocaloric cooling, a material is alternately magnetised and demagnetised.
Electrocaloric cooling is another promising approach, in which an electric field polarises and depolarises a ferroelectric material. Breaking the alignment between neighbouring electric dipoles during depolarisation costs energy and hence drops the temperature. ‘Electrocaloric devices for cooling have a compact size and light weight that are inaccessible for magnetocaloric or mechanocaloric devices,’ says Guangzu Zhang of the Huazhong University of Science and Technology (Hust).
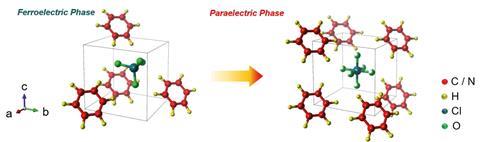
Until now, materials showing a significant electrocaloric effect have fallen into two major categories: inorganic ferroelectrics and ferroelectric polymers. But Ghangzu Zhang and his colleagues at Hust and the Beijing Institute of Technology have demonstrated the effect in imidazolium perchlorate (ImClO4), a hybrid molecular ferroelectric, making it the first in a new class of electrocaloric materials.
What’s more, this new material outperforms both of the conventional classes, in particular showing a very large electrocaloric effect with even small electric fields. This could remove the need for high operating voltages and make electrocaloric cooling more feasible in a household setting.
‘There are now many studies on electrocaloric ceramics and polymers, but molecular ferroelectrics have not been well explored, so it is exciting to see a fresh system under study,’ remarks Neil Mathur, a materials physicist at the University of Cambridge, UK.
However, because of difficulties in making direct measurements, the researchers relied on mathematical rules called the Maxwell relations to deduce the magnitude of the effect. Although a common technique in the field, it is not universally accepted.
‘This indirect method uses the polarisation from the bipolar hysteresis loops in a ferroelectric with large polarisation hysteresis, and can lead to a very wrong result,’ says Qiming Zhang, an electrocalorics expert at Penn State University in the US. ‘The Maxwell relation is derived for thermodynamically reversible material systems and processes, and should not be used in this way.’
Guangzu Zhang agrees that there might be a discrepancy between indirect and direct measurements, but emphasises that only the former is currently possible. ‘There is no instrument that can test the electrocaloric cooling effect for a thin film with substrates,’ he says. ‘So for film investigation, indirect measurement has been widely used.’
Another limitation is that while ImClO4 shows a large cooling effect in proportion to the applied electric strength, the absolute temperature change recorded is small – a little more than one Kelvin – restricting practical applications. ‘The key issue for the future is to increase the breakdown field and achieve larger electrocaloric effects,’ says Mathur.
‘This is a difficult question to answer and everybody in this field wants to know the answer,’ says Guangzu Zhang. He suggests that better understanding the relationship between the material’s microstructure and its electrocaloric properties might hold the key to this challenge.
References
W Li et al, J. Mater. Chem. A, 2020, DOI: 10.1039/d0ta05154c









No comments yet