Home health diagnostics could soon be much more useful thanks to nanopore sensors that allow direct measurement of multiple metabolites. This will let doctors monitor their patients’ health using blood, sweat and saliva samples, as well as keep an eye out for any new diseases developing.
Although biosensors integrated into electronic devices hold promise for personalised healthcare, commercial ones can only measure glucose and aren’t able to detect other biologically relevant molecules. Converting biological signals into electrical ones would not only allow direct sampling of bodily fluids but also miniaturisation of the biosensors – providing a cheap, sensitive and automated means of tracking health conditions and searching for biomarkers for diseases.
Until now a lack of selectivity for metabolites has hindered development of next-generation devices. Giovanni Maglia and colleagues at the University of Groningen in the Netherlands have overcome this problem using a membrane-spanning nanopore, already applied as a fast, low-cost method of sequencing DNA.
Instead of trying to measure metabolites by binding them – a significant challenge as most are small molecules – the team lodged specific metabolite-binding proteins within the nanopore cavity: GBP for binding glucose and SBD1 for the amino acid asparagine. When these proteins bind their substrate it causes a conformational change that alters the electrical current across the nanopore, which can then be used to provide real-time accurate read outs of the metabolites passing through the pore from bodily fluids. ‘The open and closed state of the protein is visualised as two different ionic currents,’ Maglia explains, providing distinguishable signals for glucose and asparagine.
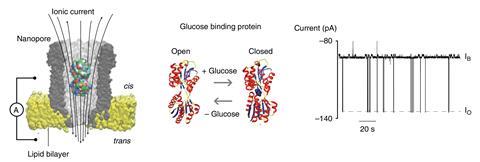
Detecting structural changes in a protein is an easier way to measure metabolites than conventional means such as detecting oxidised molecules with electrodes. The biosensor also works at the level of single molecules, bypassing the need for calibration and allowing multiple proteins to be read by a single nanopore.
Joseph Reiner, a biophysicist at Virginia Commonwealth University, US, says the work is ‘a new and important contribution to the field’. ‘Simultaneous detection of two different markers and, more importantly, in bodily fluids is really pretty tricky to do,’ he notes.
There’s also potential to use the biosensors to monitor disease – asparagine, for example, is associated with strokes and Parkinson’s disease. Because of their sensitivity and low sample preparation, ‘we can envisage a system where anyone can monitor the concentration of these metabolites in their home’, says Maglia. ‘If something is different from expected, [the device] will find anomalies that could indicate the need for further tests. As the information accumulates, we expect that these anomalies will be translated into diagnostics.’
A primary biosensor system has already been developed with the company Oxford Nanopore and could be ready in just a few months, although Maglia adds that widespread take-up will depend on interest and demand.
References
N S Galenkamp et al, Nat. Commun., 2018, DOI: 10.1038/s41467-018-06534-1














No comments yet