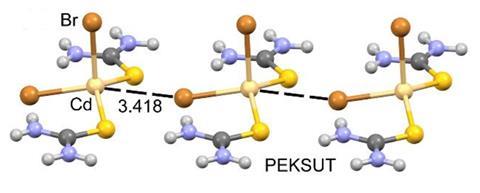
A type of non-covalent bond has been discovered in compounds of zinc, cadmium and mercury. Coined spodium bonds, they are interactions between the elements’ σ-hole – a region of positive electrostatic potential – and a Lewis base or an anion.
Spodium bonds join the growing family of σ-hole interactions among the elements on the right side of the periodic table. Common instances are chalcogen and halogen bonds. But groups 13, 14 and 15 also participate in this class of interactions called, respectively, triel, tetrel and pnictogen bonds. Within the last five years, chemists have even discovered σ-hole bonding in noble gases – aerogen bonds – and in coinage metals – regium bonds.
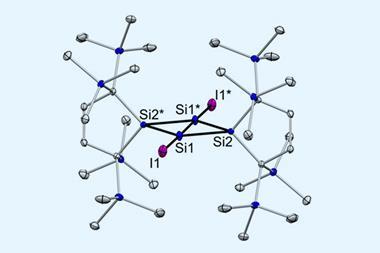
Group 12 is the latest to join the σ-hole bond crowd. Researchers had already noted spodium (Sp) elements’ tendency to participate in various non-covalent interactions, for example, anion–anion interactions between SpCl3– and cyanide. But this is the first time a systematic study has revealed weak σ-hole bonds between four-coordinate spodiums in their +2 oxidation state and electron donors such as carbon monoxide and formaldehyde.
Spodium bonds might have been previously overlooked in crystal structures as there’s significant uncertainty around these metals’ van der Waals radii. If the values are underestimated, atoms can look like they are too far apart to engage in any kind of bonding.
Through computational modelling, the team found that mercury and cadmium adducts form spodium bonds while zinc adducts mostly rely on a mix of halogen and chalcogen bonds. Natural bond order analysis showed interactions between the donor’s lone pair and an antibonding σ* orbital on the metal. This distinguishes spodium bonds from coordination bonds, which involve the metal’s d-orbitals.
Spodium bonds are weaker than similar interactions because the elements’ electrostatic σ-hole potential isn’t as strongly positive as in other elements. Nevertheless, defining such non-covalent interactions helps chemists understand self-assembly processes – like the reason halobenzenes arrange in a windmill patterns – and even control them, for example when designing solid state structures.
References
A Bauzá et al, Angew. Chem., Int. Ed., 2020, DOI: 10.1002/anie.202007814













1 Reader's comment