Scientists have surprised even themselves by creating a solid form of mercury at room temperature. They used a technique called sonochemistry to create solid nanoparticles from one of only a few elements on the periodic table to exist as a liquid at room temperature.
Sonochemistry uses sound waves to activate chemical reactions. By sonicating liquid mercury drops in water, Doron Aurbach of Bar Ilan University in Israel and his team dispersed the metal drops into nanospheres. The water also contained reduced graphene oxide so during sonication, the liquid metal emulsifies and is surrounded by layers of reduced graphene oxide. The nanoparticles became crystalline under pressure from micro-jets formed by the sonic energy-induced collapse of acoustic bubbles.
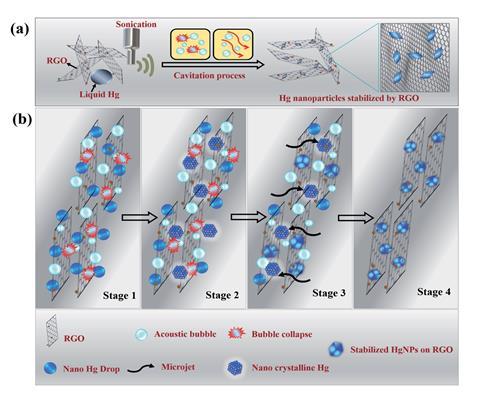
The micro-jets also propel the nanoparticles towards the graphene oxide, which has an oxidative surface and interacts with the nanoparticles on impact. This causes charge transfer between the graphene and the nanoparticles, which has a stabilising effect. The solid mercury crystals therefore become embedded within the reduced graphene oxide.
‘We discovered a new composite and maybe even a new family of composite materials’ says Aurbach. To be certain of their findings, Aurbach and his team collected extensive evidence for the new state of mercury using a variety of characterisation techniques, including thermal analysis, x-ray diffraction and x-ray absorption near-edge structure spectroscopy. This allowed them to determine that the particles consisted of solid elemental mercury.
The team tested the nanoparticles as an electrode material for the hydrogen evolution reaction. They found the electrode’s activity was comparable to other metal nanoparticles, such as palladium, ruthenium and gold, loaded onto a carbon surface.
The electrodes made by Aurbach’s team only contained a small amount of mercury. And electrocatalysts that avoid industry-standard noble metals are in demand to make processes sustainable. ‘We’ve traded precious metals for mercury, which may be very useful,’ says Aurbach.
Rajender Varma, an expert in sustainable nanocatalysis at Palacký University in the Czech Republic, comments that the work is ‘an interesting finding and may possibly stimulate further investigation’. He says future studies may involve ‘capturing or encapsulating mercury in this stabilised form’.











No comments yet