A new type of crystalline solid changes phase and reversibly releases one equivalent of solvent when heated or put under pressure. It’s ‘a unique behaviour we haven’t seen in any previous materials,’ says Michael Zdilla of Temple University in the US, part of a team that discovered the property by accident. ‘Accidents are, in my experience, the most exciting thing that can happen in science.’
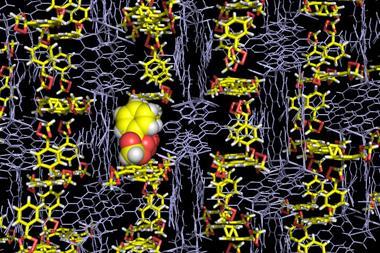
Zdilla and his colleagues were investigating solid electrolyte materials with conductivities approaching those of ceramics when they came across the unique system, which they call a solvate sponge crystal. Typically, when heating crystals, either the material melts, losing no solvent, then reforms when cooled; or it melts, and then decomposes, and the materials cannot reform on cooling. This crystal is different – rather than melting or decomposing, it changes to a different solvate.
Initially the co-crystal forms the 3:1 solvate (DMF)3NaClO4, however applying pressure releases one equivalent of DMF from the system, shifting the structure to the 2:1 solvate (DMF)2NaClO4. After releasing the pressure, the 2:1 solvate reabsorbs the DMF, reforming (DMF)3NaClO4. As the 3:1 solvate has a hexagonal crystal form, high pressure in the x and y directions deforms the crystal slightly; with ClO4- ions forced into locations originally occupied by Na+ ions, which leads to DMF being expelled from the side of the crystal – giving a thin surface layer of DMF. However when pressure is applied in the z direction, two or three Na–DMF contacts are broken and replaced with Na–ClO4 contacts, and a shift from the 3:1 solvate form to the monoclinic 2:1 solvate form, which has a molar volume that is 27% lower, is seen. This reversible shift also occurs when heating and cooling the system.

Jagadese Vittal, a crystal engineering expert at the National University of Singapore, says ‘this is a simple solvated inorganic salt crystalline material, which behaves interestingly like a sponge due to pressure and temperature changes. Further, this work opens up a new avenue for melt-castable crystalline materials for various applications.’
Further examples of this discovery ‘may lead to a new class of potential energy storage materials,’ says Zdilla’s collaborator, Prabhat Prakash, from the Indian Institute of Science Education and Research in Pune. ‘We are looking at battery applications of such materials, since these crystals have channels of sodium ions with sufficiently small energy barriers to conduct. In the present work, perchlorate and DMF both reduce easily on a typical battery anode – our plan is to discover more such crystals with more redox stable components.’
References
1 P Prakash et al, Chem. Sci., 2021, 12, 5574 (DOI: 10.1039/d0sc06455f ) (This article is open access.)
2 B Fall et al, Chem. Mater., 2019, 31, 21, 8850 (DOI: 10.1021/acs.chemmater.9b02853)

















1 Reader's comment