Gene editing enzyme ferried via endocytic pathway
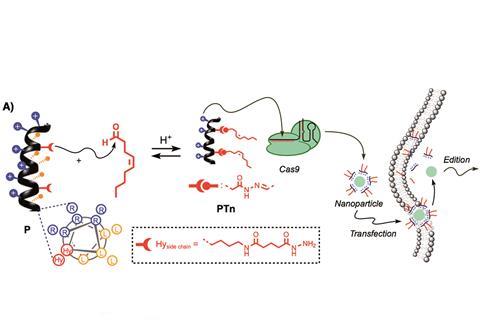
Scientists in Spain have put forward what they describe as the first non-covalent strategy for delivering the Crispr Cas9 ribonucleoprotein into cells.1
Cas9 is a large RNA-guided DNA endonuclease enzyme that is responsible for accurately recognising and cutting the desired sequence of DNA in a cell’s genome during the gene editing process known as Crispr. At the moment, Crispr scientists typically transfect cells with a plasmid containing instructions to make Cas9: however, this isn’t ideal as it might result in permanent DNA recombination and persistent expression, which could have adverse effects. Researchers are therefore exploring methods that deliver Cas9 into cells.
Javier Montenegro from the University of Santiago de Compostela and colleagues have reported a new method for delivering Cas9 directly into cells using a penetrating peptide vehicle they had previously demonstrated for gene transfection.2 The peptide vehicle consists of amphiphilic peptides connected via a hydrazone bridge to hydrophobic tails. The positive sides of the amphiphiles complex with Cas9 to form nanoparticles that the team can then transfect into cells.
The team used their peptide delivery method to edit HeLa cell genomes with a similar efficiency and less toxicity than one of the best methods reported so far.3
References
1 I Lostalé-Seijo et al, Chem. Sci., 2017, DOI: 10.1039/c7sc03918b (This paper is open access.)
2 I Louzao, R García-Fandiño and J Montenegro, J. Mat. Chem. B, 2017, 5, 4426 (DOI: 10.1039/c7tb00179g) (This paper is free to access until 13 December 2017.)
3 J A Zuris et al, Nat. Biotechnol., 2015, 33, 73 (DOI: 10.1038/nbt.3081)









No comments yet