The hyperfine structure of radium monofluoride has been measured by researchers in the US and elsewhere with such precision that the effects of the finite size of the nuclear magnetisation are visible for the first time. This enhances the possibility that researchers could use the molecule to search for add-ons to the standard model of particle physics such as signatures of dark matter or sources of matter–antimatter asymmetry.
Some theories of physics beyond the standard model predict that such signatures could be detected by tiny shifts in the frequencies of energies of certain states in atoms and molecules. Molecules are particularly suitable, partly because they are orders of magnitude more sensitive to external fields. ‘What we want in our experiments is to elongate the electric field,’ says Silviu-Marian Udrescu at Johns Hopkins University in Maryland. ‘In an atom you have to apply a huge electric field to do that because the electron cloud is very symmetrical. In a molecule the cloud is already very polarised.’
Molecules are predicted to be especially sensitive if they contain an atom whose nucleus has a rare octupole deformation, making it pear-shaped. ‘This only appears in a very few regions of the nuclear charts and all of them are radioactive,’ says Shane Wilkins at Michigan State University. This makes synthesising, cooling and probing the molecules before they decay very challenging.
In the new work, Udrescu, Wilkins and colleagues in Ronald Fernando Garcia Ruiz’s group at the Massachusetts Institute of Technology, together with international collaborators, used the collinear resonance ionisation spectroscopy setup at Cern. They bombarded a uranium carbide target with high-energy protons and then injected tetrafluoromethane gas to produce octupole-deformed nucleus radium-225 monofluoride ions. They immediately extracted these, sorting them by mass and excited them with three different lasers to measure electronic transitions in the molecules.
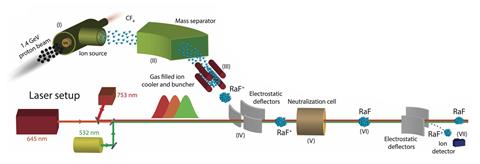
Analysis of the hyperfine structure – the splitting of the energy levels caused by couplings between electronic and nuclear spins – revealed something especially interesting. ‘In the vast majority of our experiments, we can treat the nucleus as a point-like [magnetic] dipole,’ says Wilkins. ‘The amazing thing about this molecule is that … we have a breakdown of that picture because the electron actually spends a lot of time inside the nucleus.’ The electron therefore samples the entire structure of the nucleus.
Wilkins says this effect, which has never previously been seen in a molecule, validates calculations and shows that the electron energy shifts could, in future, help to distinguish between competing models of nuclear structure. At Michigan State University, he is now working on techniques to cool the molecules, allowing higher-precision spectroscopy, which could potentially have relevance to chemistry and astrophysics.
‘It’s a superbly impressive piece of experimental work, but I would say it’s evolutionary, not revolutionary,’ says chemical physicist David Nesbitt at University of Colorado Boulder. ‘Many of these measurements have been done previously, and they’re really confirming high-level ab initio calculations at a higher level of accuracy than was heretofore achieved… I don’t see the direct connection with how this is going to challenge the standard model.’ The researchers respond that, though the molecule has been investigated previously, no previous measurements have achieved sufficient precision to resolve the hyperfine structure. ‘Without our measurements, which are the first of their kind in any molecules with half-lives this short, there are no future precision measurements investigating the fundamental symmetries of the universe in 225RaF,’ says Wilkins.
On 31 October 2025 the affiliation of Shane Wilkins was updated.
References
S G Wilkins et al, Science, 2025, 390, 386 (DOI: 10.1126/science.adm7717)














No comments yet