The charging and discharging rates of a wide range of lithium-ion batteries are better explained using model that accounts for both electron and lithium ion transfer, researchers in the US have shown. The findings, which show that ion transfer alone does not usually limit how fast a battery can charge and discharge, could be vital to future device optimisation.
The rechargeable lithium batteries that power myriad electronic devices today rely on a phenomenon known as intercalation. This involves repeated insertion and removal of lithium ions from spaces in between the atomic planes of layered electrodes during charge/discharge cycling. But the underlying reaction mechanism that governs this process remains poorly understood.
Researchers have generally assumed that the current flow is limited by the rate at which lithium ions travel between a battery’s electrode and electrolyte. This leads to the Butler–Volmer equation, which proposes an exponential relationship between the current and the overpotential. ‘The Butler–Volmer equation does not really have an underlying microscopic mechanism,’ says Martin Bazant, a chemical engineer based at the Massachusetts Institute of Technology, US. ‘It’s essentially fitted to the data with a couple of free parameters.’
In 2021, Bazant, together with MIT colleague Yang Shao-Horn and others, presented an alternative theory they called coupled ion–electron transfer. In this, the fundamental limitation is solid electrode’s ability to undergo electron transfer reactions with the solvated ions. The researchers showed that this more accurately describes the behaviour of lithium iron phosphate under charge/discharge cycling than the Butler–Volmer equation. Lithium–iron phosphate is increasingly popular as a cathode material thanks to its thermal stability relative to traditional materials such as lithium cobalt oxide, despite its lower electrical conductivity. ‘It makes sense that it would be difficult to transfer electrons into that material, so an electron transfer-limited process there is perhaps more intuitive,’ says Bazant.
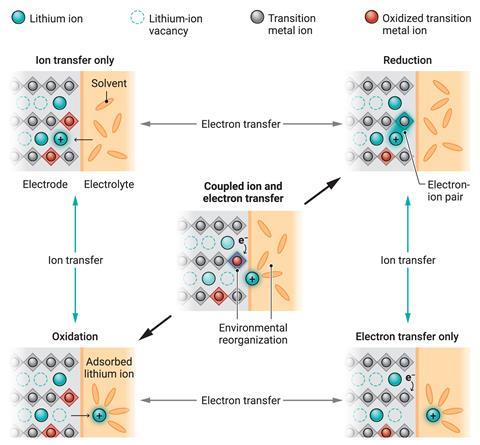
In the new work, Bazant, Shao-Horn and their colleagues tested a range of popular cathode materials such as nickel manganese cobalt and lithium cobalt oxide – most of which are good electrical conductors – are found that the predictions of coupled ion–electron transfer were still more accurate than the Butler–Volmer equation. ‘The popular battery materials we’ve studied here are, almost by definition, very good at ion transfer,’ explains Bazant.
The work could help researchers to optimise batteries in several ways. First, it reveals previously unknown effects that arise from changing the counteranion and electrolyte. Secondly, the physics underpinning the coupled ion–electron transfer model could lead to more rational design of electrode materials. ‘The mathematical framework connects the measured rates of reaction to microscopic material properties that can, in principle, be computed by quantum calculations and molecular-level simulations,’ says Bazant. ‘And that paves the way to engineering of these battery interfaces that has not been possible before.’
Alexander Urban, a computational materials scientist who works on atomic-scale simulations of electrochemical devices, believes the findings will be important to the community. ‘One of the places in which we are a little unsure of the quality of our models is … what happens when lithium is inserted into an intercalation electrode and vice versa,’ says Urban.
‘This process clearly has two contributions to it … [Bazant and his colleagues] get to the bottom of how much these contributions matter, and come to the conclusion that both are important,’ he adds. ‘That’s great for me to know because we often run these simulations without accounting for electron transfer. The next question is: how big is the error we are making?’
References
Y Zhang et al, Science, 2025, DOI: 10.1126/science.adq254
















No comments yet