A stable, selective and precious metal-free catalyst for the conversion of waste carbon dioxide into synthesis gas has been developed by researchers in the US and Canada.1 The work potentially provides a step towards the production of valuable industrial chemicals from captured carbon in future chemical refineries.

Synthesis gas, or syngas – a mixture of carbon monoxide and hydrogen – is crucial for producing numerous industrial chemicals such as ammonia, methanol and larger hydrocarbons. Today it is most commonly made either from steam reforming of methane or from gasification of coal or heavy hydrocarbons, both of which are highly endothermic processes and have large carbon footprints. An alternative is the reverse water–gas shift, in which carbon dioxide is reduced to carbon monoxide by hydrogen, while the hydrogen is oxidised to water. Using an excess of hydrogen can produce syngas.
Most industrial hydrogen is actually the product of steam reforming of methane followed by the forward water–gas shift reaction to produce more hydrogen from the carbon monoxide. Interest in renewable hydrogen produced by the electrolysis of water is growing, however. ‘Dozens of sources of CO2 like steel companies and cement companies are going to keep producing CO2 regardless of the hydrogen production cycle and somebody needs to address that,’ says Milad Ahmadi Khoshooei at Northwestern University, US. ‘The capture part is much more advanced than the conversion part, so let’s assume we capture the CO2. We need to use it instead of just sticking it underground.’
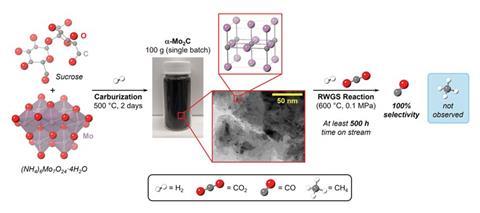
Khoshooei and colleagues at Northwestern and the University of Calgary in Canada, led by Northwestern’s Omar Farha, sought an efficient catalyst that was selective for carbon monoxide, rather than methane, under an excess of hydrogen. Previously, molybdenum carbide catalysts have shown promise, but they have often either shown insufficient initial selectivity or decayed rapidly at the high temperatures the reaction uses.
The researchers discovered from spectroscopic analysis and density functional theory simulations that the metastable cubic phase of molybdenum carbide was more active and selective than the stable hexagonal close-packed phase. They found that, simply by carburising table sugar with ammonium molybdate, they created a catalyst comprising phase-pure nano-crystallites of cubic molybdenum carbide protected by a carbonaceous layer and interstitial atoms that prevented recrystallisation. At 500°C, the catalyst showed rapid, near-equilibrium conversion and 100% selectivity to carbon monoxide. It retained this for 500 hours on stream. The researchers are further developing the catalyst towards commercialisation.
Bert Weckhuysen at the Inorganic Chemistry and Catalysis Group at Utrecht University in the Netherlands, together with colleague Eelco Vogt, this week published a detailed analysis of the challenges of creating fossil-fuel free refineries by 2050.2 Weckhuysen says the present work is ‘a mechanistically thorough study on a specially derived catalyst for an important reaction, which in future reaction scenarios could lead to carbon neutrality – or a reduced carbon footprint – in CO2 conversion processes.’
He is especially impressed by the mechanistic explanation underpinning the stability of this particular molybdenum catalyst and the researchers’ validation of it through experiment and theory. ‘I think they need to look at the influence of impurities, because many of the CO2 point sources from things like metallurgy are not just CO2: they contain other elements – metals and others. The question is if a real, stable operation can be maintained under harsh, true operating conditions.’
References
1 MA Khoshooei et al, Science, 2024, 384, 540 (DOI: 10.1126/science.adl1260)
2 ETC Vogt and BM Weckhuysen, Nature, 2024, DOI: 10.1038/s41586-024-07322-2















No comments yet