A computational study has revealed the nature of quantum tunnelling that occurs in a reaction linked to the ozone hole in Earth’s stratosphere.1 The OH + HCl → H2O + Cl reaction is important because it releases chlorine atoms, which catalyse the destruction of ozone.
Classically, a chemical reaction takes place if there is enough energy to propel it over an activation barrier, and the reaction rate is dependent on temperature following the Arrhenius formula. But in the quantum world, chemical reactions can happen at energies well below this barrier through a process called quantum tunnelling. This is exactly what happens during atmospheric and interstellar processes, where reactions take place even at extremely low temperatures.
Quantum tunnelling was already thought to play a role in the OH + HCl → H2O + Cl process – the reaction rate is essentially temperature-independent below 250K, and calculations have shown that the reaction deviates substantially from Arrhenius behaviour.2,3 However, the exact nature of this quantum tunnelling was unknown.
Now, a team around Dong Hui Zhang, from the Dalian Institute of Chemical Physics in China, has undertaken quantum dynamics calculations on newly constructed potential energy surfaces to study the reaction dynamics in detail. They find that strong hydrogen bonding causes Feshbach resonances, which are a type of quantum transition state. ‘Reaction resonances can substantially enhance tunnelling effect because they are trapped in the hydrogen bond well right before the barrier,’ Zhang explains. This means there is a higher probability that a hydrogen atom will tunnel through the activation barrier.
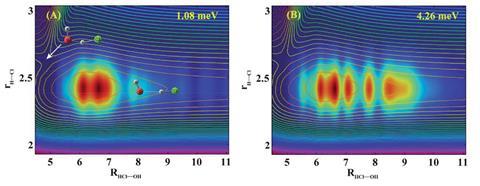
‘In fact, our quantum dynamics results were not surprising to me. Due to the existence of a strong hydrogen bond interaction between OH and HCl, there is a rather deep well in the entrance channel,’ Zhang explains. The Feshbach resonances are trapped in a bending–torsion excited state, which suggests the reaction behaves in a different way to other atmospheric processes investigated by the team, such as F + H2O,4 where strong hydrogen bonding is not present in the entrance channel.
‘[The study] demonstrates the enormous progress in the construction of accurate potential energy surfaces achieved in the last two decades,’ says Uwe Manthe, an expert in quantum transition states from the University of Bielefeld, Germany. ‘Only the combined use of accurate quantum dynamics and accurate potential energy surfaces facilitated the impressive accuracy achieved in this work.’
The researchers hope that experimentalists will undergo molecular beam studies to back up their findings, although Zhang warns ‘to my understanding, it is very challenging for the detection technologies used in experiments currently.’
References
1 X Xu et al, Chem. Sci., 2022, 13, 7955 (DOI: 10.1039/d2sc01958b)
2 Song et al, J. Phys. Chem. A, 2015, 119, 826 (DOI: 10.1021/jp512021m)
3 J Zuo et al, Phys. Chem. Chem. Phys., 2017, 19, 9770 (DOI: 10.1039/c7cp00920h)
4 Zhang et al, Nat. Commun., 2020, 11, 223 (DOI: 10.1038/s41467-019-14097-y)











No comments yet