Two conformers of the same molecule react independently to form a different rearrangement product rather than taking the much lower energy barrier pathway of interconverting into the other conformer, chemists have discovered. The unprecedented process, entirely controlled by quantum tunnelling, ‘is completely breaking the classic transition state rules’, says study leader Cláudio Nunes from the University of Coimbra, Portugal. Understanding what and how tunnelling controls chemical reactivity could be ‘a game changer for selectivity’, Nunes asserts.
‘Chemistry typically tries to control the selectivity of a reaction through competitive energy barriers between the possible products,’ explains Nunes. But this is not what happens in the case of 2-formylphenylnitrene’s syn and anti conformers. At –263°C the two conformers don’t interconvert by rotation around a single bond. Despite the isomerisation having an extremely low energy barrier, the syn and the anti conformers prefer different, separate pathways: they rearrange into a 2,1-benzisoxazole and an imino-ketene, respectively.
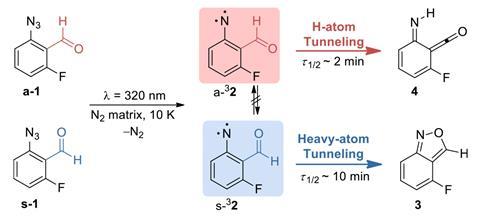
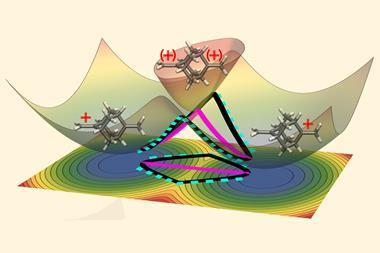
The counterintuitive process happens entirely through quantum tunnelling, meaning particles are simply sneaking through the energy barrier between reactants and products. In the case of anti 2-formylphenylnitrene, it’s hydrogen atom tunnelling, for the syn conformer it’s oxygen and nitrogen. ‘Our system shows selectivity that doesn’t depend on the energy barrier at all,’ Nunes says. ‘We have a product outcome that cannot be predicted according to the classic rules.’
For tunnelling, factors like energy barrier height – key for thermal reactivity – become irrelevant. Instead, what matters more is the energy barrier’s width, which is much larger for the isomerisation than for the rearrangement reactions. Similar tunnelling controlled reactions were first described by Peter Schreiner’s team from the University of Giessen in Germany. But dual tunnelling control is a new type that complicates the picture.
‘The individual and concurrent reactivity of two conformers through two tunnelling pathways … is new and highly remarkable,’ Schreiner comments. ‘The reaction outcome cannot be understood on the commonly accepted grounds of kinetic versus thermodynamic control. Only tunnelling control can help rationalise the observations made.’
The main takeaway for chemists, says Schreiner, is that ‘tunnelling control indeed is the third reactivity paradigm to understand chemical reactions’ and that has ‘clear implications for synthetic reaction planning’. ‘We can start to think about designing systems that can be controlled by tunnelling,’ adds Nunes. ‘It opens perspectives to look at reactivity a different way.’ Although at higher temperatures tunnelling may not be the main driving force for processes, even a small tunnelling contribution can influence reaction outcomes.
Nunes and his colleagues are now investigating reactions under conditions that approach conventional lab settings. This means carrying out reactions in liquids rather than in frozen solid gases, and higher temperatures of around –100°C.
References
CM Nunes et al, J. Am. Chem. Soc.,. 2022, DOI: 10.1021/jacs.2c09026














3 readers' comments