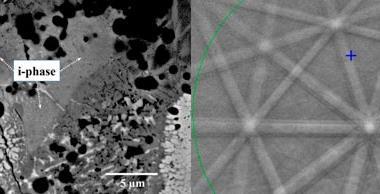
The first experimental observation of quasicrystal growth has been conducted in aluminium–nickel–cobalt by researchers in Japan. Instead of growing in a perfect state, these crystals form with frequent errors at the growth front, which are then corrected later. The researchers are unsure how this occurs, but they believe it does not follow the standard theoretical model of quasicrystal growth.
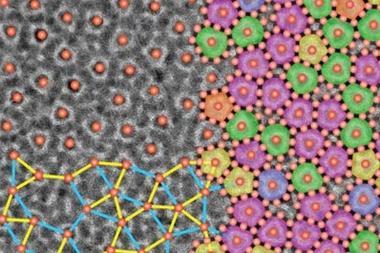
Quasicrystals, whose 1984 discovery was rewarded with the 2011 chemistry Nobel prize, have atomic clusters held in place as in a crystal, but instead of having a simple, repeating unit cell, the structure follows more complex, global mathematical rules. This poses a puzzle: if atoms can sense and respond to only their local environment, how can they assemble according to global mathematical rules? In 1988, Paul Steinhardt of the University of Pennsylvania, US, and colleagues proposed a scheme to explain this, and many researchers believed it must be correct.
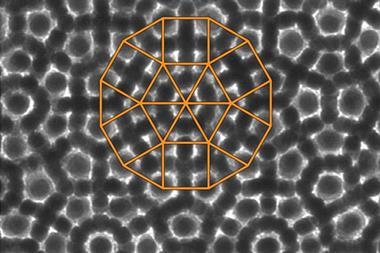
In the new work, Keiichi Edagawa of the University of Tokyo and colleagues heated a thin sample of the quasicrystalline alloy Al70.8Ni19.7Co9.5 to 1183K – high enough to induce re-crystallisation – and held it at this temperature. They monitored the crystal structure's evolution using high-resolution transmission electron microscopy. As crystals grew, atoms often entered in the wrong place or with lattice spacings violating the mathematical rules governing quasicrystalline order. These errors, however, were repaired later when atomic clusters changed position, often after several further layers had grown on top of the layer containing the error. The physical mechanism underlying this repair process is not yet clear, but Edagawa says: 'In our case, the quasicrystals do not grow according to Steinhardt's model.'
Primoz Ziherl of the Jozef Stefan Institute in Slovenia believes the most important conclusion is that perfect quasicrystals can self-assemble without the atoms slotting into prearranged slots, implying that non-local forces are unnecessary. 'These results were not entirely unexpected, but nevertheless it's very, very reassuring,' says Ziherl.
Steinhardt agrees, saying it is an 'important development to empirically verify that perfect growth is possible'. He takes issue, however, with the researchers' contention that the formation observed does not obey his group's mechanism. 'Our rules were set for a mathematical abstraction of tiles,' he explains. 'They are now applying the principle to an alloy of three types of atoms. You would never expect things to be exactly the same.' He also points out that the carefully-controlled conditions of the experiment, in which crystals were constrained to grow in one direction, deviate considerably from the general rules underlying his group's model. To address this, the researchers are now planning to form crystals by standard industrial processes and observe the crystals produced to determine the directions in which the grains have grown.

















No comments yet