An electrochemical hydrogenation reaction could be a practical new tool for laboratory chemists. The technique eliminates the need for the hydrogen gas and expensive transition metal catalysts that are typically used in hydrogenations. It is also tolerant of many different functional groups, making it well suited for late-stage chemistry.
Hydrogenation reactions are a mainstay of synthetic chemistry, but they usually require catalysts based on rare and expensive metals like ruthenium, rhodium or iridium. These reactions also tend to use harsh reaction conditions, including high pressures of hydrogen gas. Over the last decade electrochemical alternatives have been developed, but these have faced challenges, in particular the limited scope of substrates that can be used.
Now, a team led by researchers at the University of Greenwich, UK, has developed a new electrochemical hydrogenation protocol, based on the generation of diimide from hydrazine hydrate, which can be used with a wide range of starting materials. The process enables the reduction of akenes, alkynes, as well as compounds bearing nitro and azido groups. Due to the method’s simplicity and its tolerance of different substrates, the researchers behind the work believe it will be a useful tool for medicinal chemists needing late-stage hydrogenation for their compounds.
Greenwich’s Kevin Lam led the study alongside academic colleagues in Italy and researchers from GSK’s Medicines Research Centre in Stevenage. Lam says the research could help synthetic chemists who don’t have practical access to hydrogen gas. ‘For medicinal chemists on a small scale, this method could be applied,’ says Lam. ‘In some cases the selectivity is even better than using palladium and hydrogen under high pressures.’
Greenwich-based researcher Jamie Walsh, who also worked on the project, describes the method as ‘really easy and simple’ to carry out. ‘You take your electrochemical cell and place your compound in the solvent, which could be methanol or acetonitrile, add the hydrazine hydrate and start the electrolysis,’ he explains. ‘Sometimes you can add some trifluoracetic acid if your compound bears and easily reducible group, but it’s a really simple reaction.’
The process begins with the oxidation of the hydrazine at the anode to form diimide. This diimide can then donate its hydrogen atoms to reduce the substrate compound, with the reaction being driven forward by the formation of dinitrogen, which is given off as a gas.
Lam acknowledges that using hydrazine – a component of rocket fuels – in a reaction might raise eyebrows. ‘On a large-scale using hydrazine can be problematic, because it is explosive,’ he says. ‘In our case it’s only used on a small scale and we are using hydrazine hydrate, so it is a lot easier to handle.’
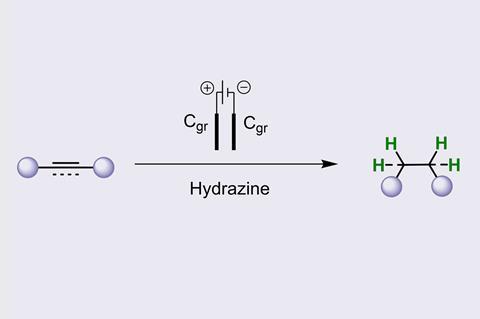
The group also used the technique for a series of deuteration reactions. ‘Deuterated compounds can have increased pharmacokinetic properties, which is why they can be important for pharmaceutical applications.’ says Walsh. By adding heavy water (D2O) to their solvents, the team were able to deuterate compounds while bypassing the need for expensive D2 gas. Some of the alkane products generated showed a deuterium incorporation higher than 99%.
Philip Norcott, from Australian National University in Canberra, an expert in organic synthesis, says the work can ‘provide new opportunities for chemoselectivity in chemical reactions’.
‘Hydrazine remains a toxic starting material, but one of the benefits of electrochemical handling is the mild reaction conditions using ambient temperatures and pressures, and that the reaction rate can be controlled simply by the set current flow,’ he adds.
References
C Russo et al, Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202309563













No comments yet