The mystery behind why the world’s first high explosive, fulminating gold, produces a purple smoke when it detonates has been solved, finally resolving a 400-year-old alchemical puzzle.
Fulminating gold is not actually a chemical fulminate, a compound containing the ion CNO-, such as mercury(II) fulminate or silver fulminate. Instead, it is a mixture of a number of different compounds with gold(III) compounds complexed with ammonia providing most of the explosive punch. Its name comes from its explosive nature, from the Latin fulmen, or lightning. It was first mentioned by alchemists in the 16th century, and described by the German alchemist Sebald Schwaertzer, purportedly in 1585, to create ‘a beautiful, purple-coloured Aurum Fulminans’ within four days.
Over time, fulminating gold became the subject of several myths, including that the explosion was only directed downward, attracting the interest of Europe’s scientists. Major figures of the 17th and 18th century – including Robert Hooke, Carl Wilhelm Scheele and Antoine Lavoisier – all studied it, and it even temporarily blinded Jöns Jakob Berzelius when he dropped a breaker containing a sample in 1809, resulting in glass shards in his eye and permanent, purple-coloured scars on his hand. However, while the chemistry of the fulminating gold recipe has been understood for centuries, one question has lingered: why does its detonation produce purple smoke?
Now, Simon Hall’s team at the University of Bristol has discovered the answer. The most common explanation for the purple smoke is that it contains gold nanoparticles. These metal nanoparticles are able to host an unusual phenomenon as a result of their size: wave-like oscillations in their electron clouds. These quasiparticles, known as surface plasmons, can interact with incoming light absorbing some wavelengths and reflecting others that have a longer wavelength than the oscillations in the electron cloud. However, this had never been confirmed experimentally. To do so, the team created fulminating gold, then detonated 5mg samples on aluminium foil by heating it; they then captured the smoke using copper meshes, and investigated what they had caught using transmission electron microscopy.
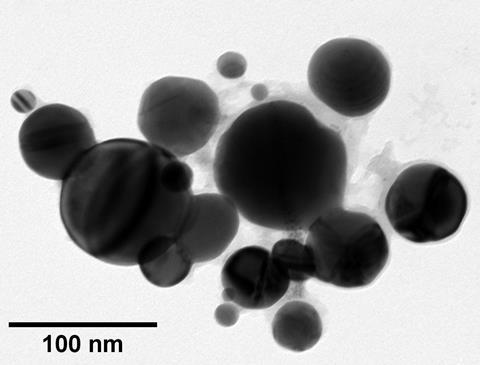
Sure enough, the team discovered the smoke contained spherical gold nanoparticles ranging in size from 30nm to 300nm, confirming the theory that the gold was playing a role in the mysterious smoke. The particles’ presence also explains many of the other mysteries of the explosive, including why Berzelius’ scars were purple (the wavelength of violet light is about 400nm), and how the explosive was used by alchemists to coat objects in a purple patina.
‘The work confirms the long-held assumption that the purple fumes of fulminating gold contain elemental gold in the form of nanoparticles,’ confirms Curt Wentrup, an organic chemist at the University of Queensland, who has researched the topic in the past.
However, he adds, there is still one final mystery about fulminating gold: who discovered it? Although Schwaertzer’s Chyrosopoeia Schwaertzeriana claimed it was written about in 1585, it was only published in 1718. Instead, Wentrup adds, the real originator was an alchemist (probably a German salt maker called Johann Thölde) writing under the pseudonym of a monk called Basil Valentine, who published the recipe in 1599 in his Twelve Keys of Basil Valentine. Schwaertzer, Wentrup adds, ‘clearly refers to Basil Valentine’ in his work – something not possible if he had really published his book 14 years earlier.















No comments yet