By contorting a molecule contributing to orange peel’s great smell into different shapes, US researchers have served up new hints about how smell works. Kevin Ryan’s team at the City College of New York explored how molecules modified from classic citrus odorant octanal fit into and activate an olfactory receptor. They occupy the receptor like keys in a lock – but then send weaker or stronger signals to our brains.
This receptor recognises a preferred octanal conformation, Ryan’s team has shown. He suggests that this receptor binds various aldehydes, ‘then waits to see what shapes the ligand chain can adopt before reporting to the brain’, he tells Chemistry World. ‘That’s the little piece of chemical space that this receptor has evolved to watch over,’ he says.
Smell is clearly an important sense, as around half of the human genes that encode G protein-coupled receptors (GPCR) are for olfactory receptors. But it remains surprisingly mysterious, as scientists have been unable to solve the structures of any mammalian olfactory receptors. Without this knowledge, it’s even more important to relate chemical structures to how they smell.
Ryan has worked on this question since 2008, focusing on the OR-I7 receptor that recognises aldehydes with 7–10 carbons. His team had previously shown that u-shaped cyclohexylethanal bound to OR-I7 receptors but didn’t activate them. Ryan continued to think about the exact role that shapes play in how OR-I7 registers smells. He thought that perhaps cyclohexylethanal wasn’t long enough to fully mimic octanal, so his team added an ethyl group to make 2-(4-ethylcyclohexyl)ethanal. Protruding groups at either end of this compound also mean that this compound forms cis- and trans-stereoisomers that mimic different octanal conformations.
Smell chemicals bind too weakly to OR-I7 to measure directly, so PhD student Min Ting Liu used a different strategy. Molecules like octanal stabilise the active OR-I7 conformation, starting events leading to calcium influx into the neuron, initiating a nerve signal to the brain. Ryan, Liu and colleagues therefore isolated neurons from mice, and used a fluorescent molecule that recognises calcium to detect when smells activate OR-I7. In doing so, they found that both stereoisomers of 2-(4-ethylcyclohexyl)ethanal bound to OR-I7. The cis-isomer only caused half the neuron fluorescence that octanal would. By contrast, the trans-isomer could cause around 20% more fluorescence than octanal.
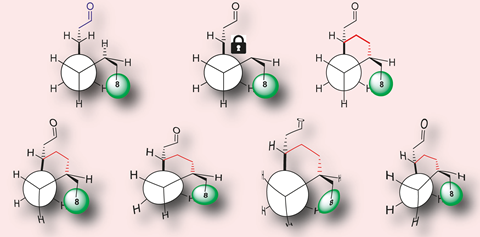
These findings show the importance of conformational shape in triggering olfactory receptors. However, despite octanal’s citrus-like fragrance, trans-2-(4-ethylcyclohexyl)ethanal doesn’t create a super-citrus smell, Ryan emphasises. That’s because when we smell octanal the overall smell ‘code’ involves many different olfactory receptors, and many chemicals comprise the smell of citrus. OR-I7 ‘contributes to the overall code but doesn’t necessarily dominate it’, Ryan stresses.
Flavour chemistry expert Jane Parker from the University of Reading, UK, calls the work ‘fascinating’. ‘The novelty lies in demonstrating that OR-I7 uses information from the whole molecule to determine whether an activation signal is sent to the brain,’ she says. ‘This work shows the importance of the conformation of the non-polar hydrocarbon chain, which dictates the efficacy of the activation signal.’















No comments yet