Thanks to riboflavin, chemists see route to Z-isomers of α,β-unsaturated carbonyl intermediates
By looking deep into our eyes – particularly the chemicals that help us see – researchers in Germany have discovered a cheap, easy route to useful but hard-to-make organic compounds.
The University of Münster chemists used riboflavin, also known as vitamin B2, to catalyse certain alkenes to selectively form Z-isomers that thermodynamics normally render difficult to get. So far, Ryan Gilmour and Jan Metternich have shown the ultraviolet light-driven approach works on a,ß-unsaturated carbonyl compounds, intermediates for targets in drug discovery or agrochemical research. Gilmour hopes the scope can be broadened using similarly inexpensive alternatives to riboflavin, which cost the team $27.50 (£18) for 25g. ‘We’d love to create a generic toolkit of commercially-available catalysts,’ Gilmour tells Chemistry World.
Gilmour and Metternich’s discovery builds on riboflavin’s role catalysing structural changes in the alkene retinal. This molecule’s transformation from its Z-form, with bulkier groups on the same side of double bonds, to its E-form, where they’re on opposite sides, helps our eyes detect light. Gilmour came upon this process when hunting inspiration to develop inexpensive organic catalysts for the E/Z-transition, as chemists have few routes to Z-alkenes. Noting that a 1967 study of visual biochemistry mentioned that riboflavin helps retinal switch back to its Z-form, he recommended Metternich explored its synthetic use.
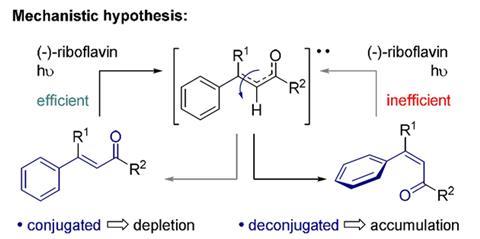
The reaction is also easy because riboflavin isn’t very soluble. The conversion can consequently be done by dissolving the alkene, adding riboflavin, shining UV light on it, filtering out riboflavin, and evaporating solvent. Gilmour says that the reaction is already causing excitement amongst people who use a,ß-unsaturated carbonyls for palladium couplings.
‘It's a superb piece of work, and the method will hopefully become widely applied,’ comments Rafal Klajn from the Weizmann Institute of Science in Rehovot, Israel. He hopes that the approach could also inspire the development of new molecular switches.












No comments yet