Lemon juice both biocatalyst and solvent in the synthesis of organic compounds
Lemon juice turns out to be a green alternative to hazardous solvents and metal catalysts in the preparation of bioactive molecules.
Synthesising quinoxalines and benzoxazines, compound families with vast applications in medicinal and material chemistry, usually requires heavy metal catalysts and harmful solvents like toluene or xylene. A group of Serbian scientists led by Nenad Janković from the University of Kragujevac used lemon juice to make these compounds from substituted ethyl 4-oxo-2-butenoates and ortho-phenylenediamine or ortho-aminophenol with outstanding atom economy and excellent yields.
The citric acid in the juice catalyses the formation of a reactive species from the ethyl 4-oxo-2-butenoate derivative. When the reaction is finished, the product, insoluble in water, precipitates.
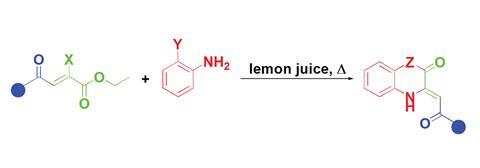
The researchers found that using enolate salts as starting materials, instead of their respective enols, speeds up the process, making it 12 times faster. Moreover, they could recycle the lemon juice up to five times without any loss in catalytic activity.
Janković’s team also managed to prepare some of the bioactive derivatives in a multi-gram scale, thus showing the scalability of the process.
This article has been edited on 11 November to correct the nature of the reactive species in the reaction.
References
This article is free to access until 04 January 2017
J Petronijević et al, Green Chem., 2016, DOI: 10.1039/c6gc02893d
















No comments yet