An electrochemical reaction that selectively oxidises primary and secondary alcohols into their corresponding aldehydes and ketones at room temperature offers a contemporary twist on the classic Swern oxidation. This electrified version of the organic chemistry textbook staple, which uses dimethyl sulfide as a redox mediator, can be scaled up in flow conditions without risking over-oxidation, and avoids the cryogenic temperatures and hazardous reagents required by its predecessor.
‘One of the key intermediates in the Swern mechanism looked suspiciously like something that could be generated by electrochemistry, that’s where the idea came from,’ explains Simon Kaltenberger, a visiting PhD student in Kevin Lam’s group at the University of Greenwich, UK.
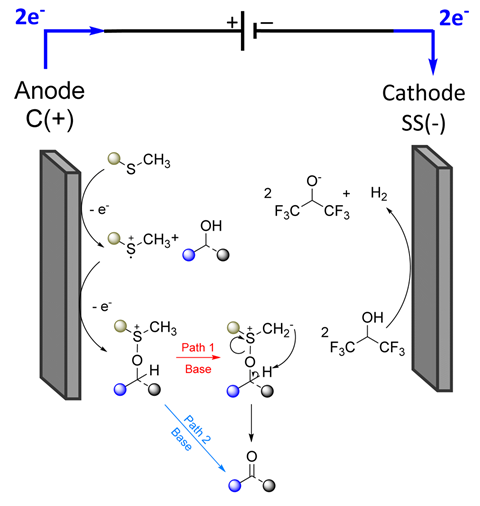
Traditional oxidation methods – which use chromium(vi) reagents or Dess–Martin periodinane – and the Swern oxidation require low temperatures, are highly sensitive and are often difficult to scale up. The method developed by Lam and co-workers, bypasses these limitations by performing an anodic oxidation under ambient conditions in an undivided cell, converting a thioether, via its radical cation form, into the oxidising agent. The method obtains good yields and broad functional tolerance.
The reaction also features an element of modularity where ‘you can use the mediator you like depending on what you want’, explains Lam. ‘Either use a simple thioether, which removes the need for a column to purify the product or use a safer less volatile thioether. It’s flexible and versatile for people to use.’
Looking ahead, the team is focused on scaling the method beyond the lab. ‘The main focus for this project was to make [this reaction] scalable which we’ve done on a lab scale,’ says Conall Molloy, a PhD student in Lam’s group. ‘I’m going to GSK to actually try and implement this on an industrial scale.’
An industrial translation will have its own challenges notes Siegfried Waldvogel, director of the electrosynthesis department at the Max Planck Institute for Chemical Energy Conversion and chief technology officer at ESy-Labs in Germany. ‘In technical applications, down-stream processing takes much more effort … you would need a way to easily recycle the total electrolyte system, otherwise it will be difficult.’
A promising solution is flow chemistry, which could remove the need for an electrolyte system. ‘When scaling up in batch, you see over-oxidation which is difficult to separate from the aldehyde product in a lot of the cases,’ Molloy explains, ‘but in flow, you don’t really have that problem.’
Mahito Atobe, an electrosynthesis expert from Yokohama National University in Japan says the work is ‘a groundbreaking new synthetic approach that provides a safe and sustainable alternative for both laboratory and industrial applications’.
‘It’s great that people read old literature, get inspired by that and bring ideas together, to make something really creative out of it,’ adds Waldvogel.
Additional information
Nour Tanbouza completed her PhD in Kevin Lam’s lab as a visiting researcher but was not involved in this research.











No comments yet