Modular strategy opens up new way to synthesise arylated thiophene rings
Approach holds potential for creating new drugs and unique optoelectronic materials
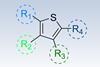
Arylating at every position on a thiophene ring has now been made easier with a modular synthetic strategy. The researchers hope this offers new ways to synthesise novel thiophene–containing drugs and polymer materials.
Thiophenes are widely used motifs in drugs and many organic semiconductor materials, the latter due to thiophene’s conjugated system allowing for efficient charge transfer. The degree of arylation on the thiophene ring is thought to help aid both solubility of drugs and tune optoelectronic properties.
