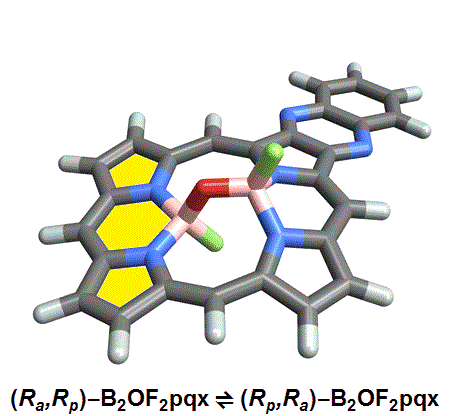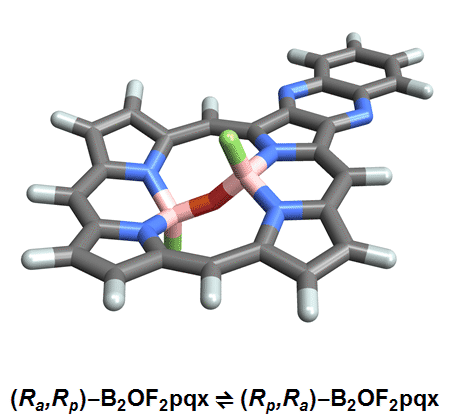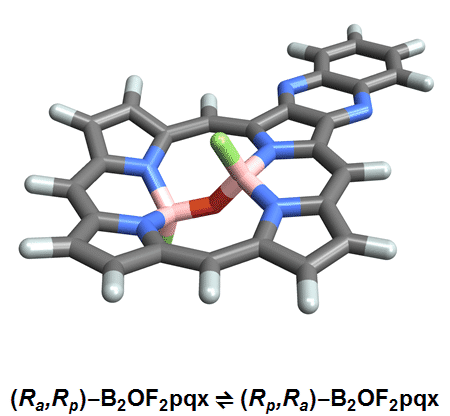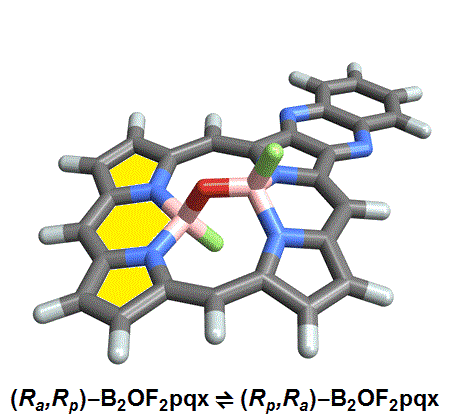
Almost 170 years after Louis Pasteur discovered chirality by picking apart left- and right-handed tartaric acid crystals under the microscope, an entirely new form of stereoisomerism has been found in porphyrin–boron complexes. These isomers, based on hindered bond angle inversion, could make for unconventional drugs or find use in molecular computers. They are the last kind of isomer that will ever be discovered, according to the chemists behind the work.
After Pasteur’s 1848 discovery – a phenomenon Lord Kelvin dubbed ‘chirality’ – it took more than 60 years for chemists to realise there is more to stereoisomers than asymmetric carbons – a carbon connected to four different substituents. A distinct type of stereoisomerism was first hypothesised in 1914 and finally confirmed in 1922. Called atropisomerism, it is characterised by either a chiral axis or a chiral plane caused by hindered rotation around a single bond. What started off as an oddity has become an established phenomenon; atropisomers have found applications as antibiotics and ligands for stereoselective reactions.
The 1960s spelled another good decade for isomerism: in 1961, chemists realised they could make molecules with chiral phosphorus centres. Nitrogen-centred chirality followed in 1969. Usually nitrogen compounds undergo pyramidal inversion, akin to an umbrella flipping inside out. This process is extremely fast, which makes it impossible to separate the enantiomers. By confining the nitrogen within a three-membered heterocycle, chemists managed to make the first isolable aziridine enantiomers.
More than half a century after these discoveries, chemists have now stumbled upon yet another fundamental type of isomerism. A team led by Jeffrey Reimers, from Shanghai University, China, and the University of Technology Sydney, Australia, and Maxwell Crossley, from the University of Sydney, Australia, has found the first example of chirality due to hindered bond angle inversion. Dubbed akamptisomerism, from the Greek work for inflexible, the discovery might be useful in molecular computing or to produce new drugs.
The emerald chiral
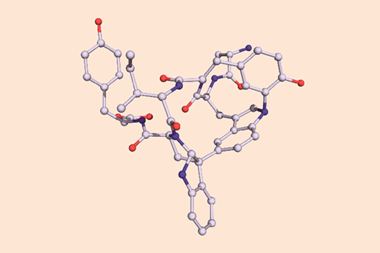
The chemists discovered akamptisomers while working on porphyrin–diboron complexes for electronic devices. When they installed a diboron bridge in the centre of a quinoxalinoporphyrin – an asymmetric macrocycle – the reaction produced not one, but four emerald green compounds. ‘We isolated two enantiomeric pairs of compounds. They were diastereomers of each other,’ explains Crossley.
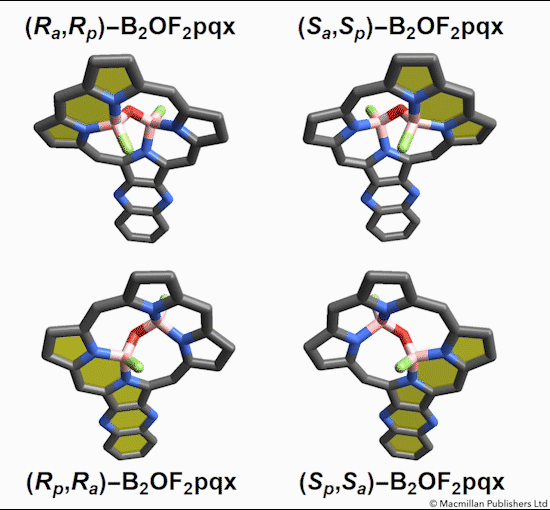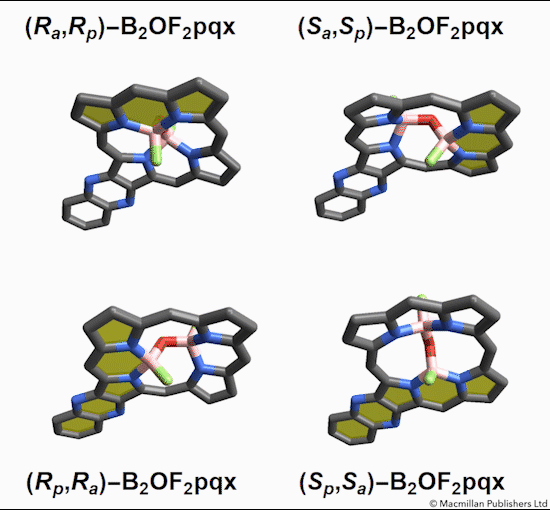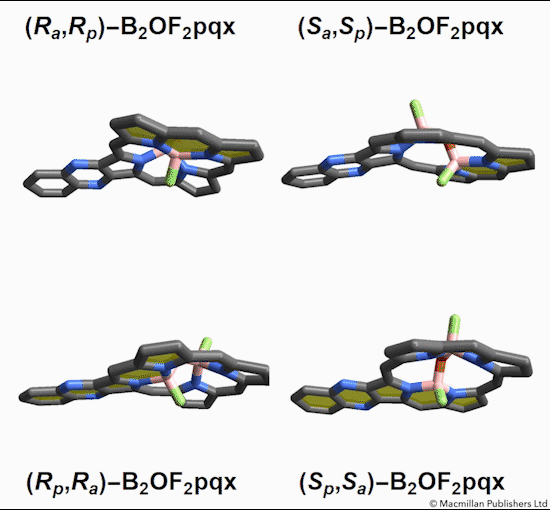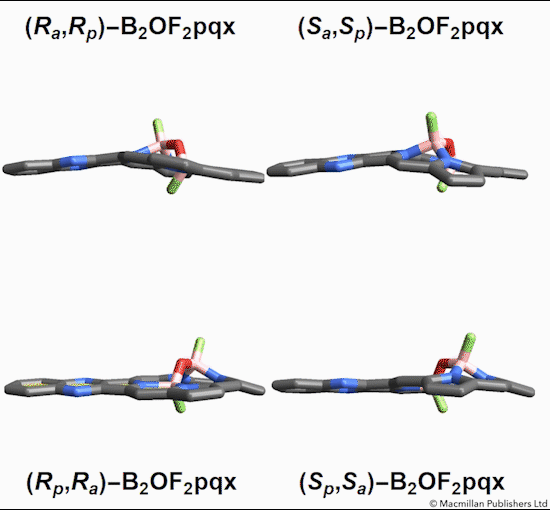
‘Nothing in Iupac’s Gold [compendium of chemical terminology] or Red Book [inorganic chemistry nomenclature] enabled us to describe the stereochemistry in our new compounds,’ Crossley says. So the team came up with its own naming system using parvo and amplo to describe the angles around the chirality-defining oxygen–boron bridge. Parvo means small in Latin, while amplo means large. ‘We looked at hundreds of [possible] compounds to make sure we can name them,’ Reimers says. ‘
Heating to around 50°C turns one diastereomer into the other through bond angle inversion – a process fundamentally different to all other types of stereoisomer interconversion. The oxygen pushes through the porphyrin’s plane via a transition state in which the boron–oxygen–boron group is linear. Usually, molecules with a bent bond interconvert through double torsion, which is too fast for enantiomers to be isolated. The akamptisomers’ porphyrin ring, however, makes torsion impossible, and the bond angle inversion is slow enough to make it possible to isolate isomers. ‘I think everyone overlooked [akamptisomerism] because it’s so simple,’ Crossley tells Chemistry World.
Akamptisomerism is also ‘the last fundamental form of conformational isomerism that will ever be discovered’, says Crossley. Having explored the mathematics behind isomerism, the team didn’t find another distinct type of interconversion.
Computer switches
‘It’s a nice and important study because it’s adding to our understanding of a fundamental principle in chemistry,’ comments Ivan Aprahamian, who works on molecular switches at Dartmouth College, US. Eventually, he thinks, this concept might make it into the textbooks. ‘One of the things that will be interesting to see is if this is a general and prevalent phenomenon or if this is an engineered phenomenon where it will happen only in very precisely designed structures.’
Reimers and Crossley think that akamptisomers might find applications in molecular computing, which needs molecules that can be switched between two states. Aprahamian suggests: ‘If you can interconvert these diastereomers and there is some interesting difference in their properties – in the case of the porphyrins this might be photophysical properties – that would be something useful that could be exploited.’
‘We’re now in the process of making different porphyrin analogues and a whole range of related macrocycles,’ adds Crossley. ‘We also have some acyclic compounds – there, the key is to hold the atoms at the right distances to allow the [akamptisomerisation] process to occur.’ The team hopes their work will encourage others to think more about how their molecules interconvert – and if they might indeed be akamptisomers. ‘We expect a lot more examples to emerge,’ says Crossley.
References
P J Canfield et al,Nat. Chem., 2018, DOI: 10.1038/s41557-018-0043-6







No comments yet