Side products might not be what you want, but they could be what you need
Organic chemists have dreamed of ideal syntheses for decades. Over the years, the vision has changed – from early ideas of a ‘beautiful’ synthesis where simple elegance was the goal, to today where more quantitative metrics are used to determine a route’s ideality.
One of the simplest goals is to obtain near-quantitative yields throughout the route – turning every bit of starting material into product. Another is to avoid purification steps, which create waste (and can also dent yields in the case of chromatographic separation with close-running impurities). In short, a reaction that converts a pure starting material completely to the desired product, without extensive purification steps, is perfect. And often we’d happily settle for just partial conversion.
Unfortunately, we seldom get this in the real world, because the real world has side reactions. These scupper your schemes by both decreasing the yield and creating the need for time-consuming and waste-producing purification steps.
Side-products are not to be confused with byproducts. Technically, or perhaps pedantically, a side-product is an unwanted alternative product formed under the reaction conditions, in addition to the one which is desired. They are offshoots of the productive mechanism.
Byproducts, on the other hand, are a necessary consequence of the mechanism. As an example, the Swern oxidation converts an alcohol into an aldehyde or a ketone (Figure 1). The reagents, dimethyl sulfoxide and oxalyl chloride, react together to form the active electrophile, giving off carbon monoxide and carbon dioxide as necessary byproducts. To obtain the desired product, the alcohol then reacts with the electrophile, and the intermediate then undergoes elimination to afford the desired carbonyl compound, along with another necessary byproduct, smelly dimethyl sulfide.
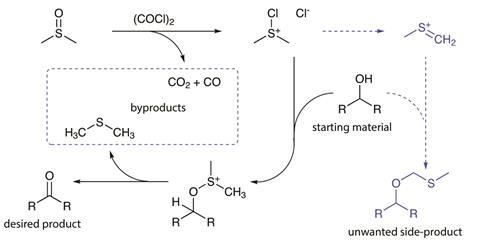
However, the electrophile can also decompose to a methylenesulfonium, particularly if the temperature isn’t low enough or reagents are added too quickly. And if this captures the alcohol, you have a side-product.
Most of the time, side reactions feel like an inconvenience. However, from the moment we start as students in an organic lab, we are told that meticulous characterisation of side-products is a virtue. Serendipitous discoveries, including entire branches of academic groups’ work, have been inspired by unexpected impurities.
Even if they don’t make your career, side-products can make a big difference to your understanding. For example, they can provide a glimpse into critical kinetic parameters if the slow step of a reaction is being outcompeted by an unwanted pathway. Likewise, for reactions with unknown or uncertain mechanisms, they can hint at the true story – seeing the homocoupling product rather than cross-coupling can suggest it’s worth trying a different ligand.
Recently, I identified a major side-product in a mesylate displacement and found that it was the result of an elimination rather than a substitution. I thought back to what I taught my undergraduate students about promoting substitution over elimination: keep the temperature cold, the conditions dilute and the solvent polar. Using my knowledge of the side-product, I switched to a low-molarity, polar N,N-dimethylformamide and water mixture at −20°C, and my yield of the substitution product shot up from 35% to a gram-scale-proof 89%.
Yet despite their usefulness, side-products have traditionally remained unreported in the primary literature. And in the modern era of data science, we have reason to not only carry out a crude mixture analysis, but to report it. As artificial intelligence software (AI) starts to mine public and private reaction databases to unearth the chemical reactions of the future, careful reporting of major side-products allows AI to account for regioselectivity, for example. This is essential for retrosynthesis planning software, particularly where C–H bonds are being replaced.
Unfortunately, complete characterisation takes time, and often does not confer any immediate benefit. Sometimes, you dutifully track down an impurity in a crude mixture, only to discover it is the reaction product of an impurity in your commercial starting material. Perhaps more often than not, I find my pure intentions for full characterisation of crude mixtures leave me no better off than when I started. I’m beginning to realise that many people who advocate characterisation for every reaction tend not to have worked in the lab for a decade or two. Still, my experience with the mesylate displacement shows that even though an ideal synthesis affords zero side-products, when they do appear in the real world, they can sometimes transform our understanding of the reactions we run.












No comments yet