The Nobel-winning invention that changed electrochemistry
Jaroslav Heyrovský
Czech chemist (1890–1967). Nobel prize winner for the invention of polarography
I remember a practical I did as an undergraduate. I have never forgotten the strangeness of the apparatus, which included a small burette filled with mercury, droplets of which fell through a solution at regular intervals, tic, tic, tic. I had no idea of the significance of the technique – polarography – at the time, but it revolutionised electrochemistry.
The 19th century is the time when electricity shifted from a being a strange mystery to take over almost every aspect of the scientific laboratory. After Alessandro Volta’s invention of his pile (Chemistry World, June 2011, p58) it was transformed by Humphry Davy and others into a tool to tear matter apart into its components. After Michael Faraday linked electricity quantitatively with chemistry, the search turned to trying to understand how electrical currents could decompose solutions. Walther Nernst and Hermann von Helmholtz coined the term ‘electrode potential’ and developed the link between concentration and the potential itself; Nernst expressed the hope that electrochemistry would one day become as simple and useful as spectroscopy. Chemists like Max Le Blanc (Chemistry World, January 2015, p39) to begin to plot ‘polarisation’ curves to investigate the electrochemical processes – his invention of the hydrogen electrode would anchor all measurements to a common standard.
But measurements in solution remained difficult and dogged by problems of reproducibility. These issues would be swept away by a young Czech postgraduate student, Jaroslav Heyrovský. Hooked on science since childhood, he started a degree at Prague’s Charles University, where his father was rector. Heyrovský was soon so excited by the latest discoveries that he begged his father to let him study at University College, London (UCL), UK, where William Ramsay had been discovering the noble gases. But Heyrovský’s dream of working with Ramsay crumbled; the year he graduated, 1913, Ramsay retired.
Heyrovský was a true Anglophile; he wrote his notebooks in English to the end of his life. Awarded a demonstratorship (a fixed term lectureship) at UCL, he made plans to adopt British nationality and began to work with the newly appointed Frederick Donnan, whose recent paper on membrane potentials had cemented his academic reputation.
Donnan suggested that Heyrovský determine the reduction potential of aluminium. The oxide layer on the surface meant that its position in the ‘electropositivity’ series had been a matter of speculation and debate – some measurements placed it as high as zinc or magnesium, others suggested it was as low as iron or even lead. With the pure metal giving inconsistent results, Donnan had been using mercury capillary electrodes similar to those developed by Gabriel Lipmann (Chemistry World, September 2015, p38). He thought that by using mercury amalgam, as Gilbert Lewis had done for the alkali metals, it would be possible to obtain a fresh surface, free of an oxide layer.
Heyrovský used a platinum–hydrogen reference electrode, using the stream of hydrogen bubbles to stir the solution and maintain an ‘inert’ atmosphere around his amalgam electrode. He established not only the reduction potential of aluminium but also gained insights into the speciation of aluminium in water by making measurements across a wide pH range.
Heyrovský was on holiday in Bohemia when the first world war broke out in 1914, and he found himself stuck in the Austro-Hungarian empire. For a few months he managed to work at the Chemical Institute in Prague, but was called up to serve in the Austro-Hungarian army. Luckily, he was appointed as a pharmacist in a hospital – a job that left him time to write up his work.
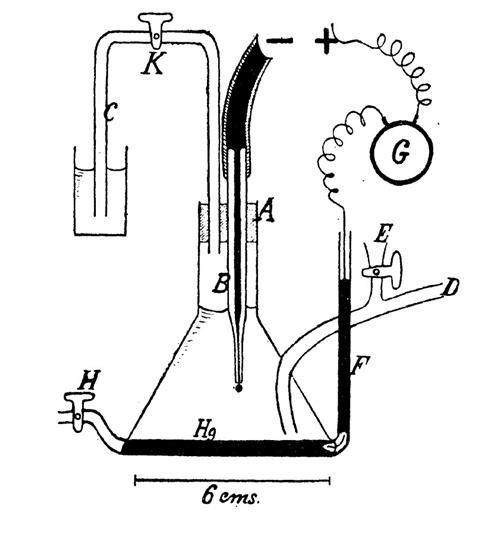
When the war ended in 1918 he submitted a thesis to Charles-Ferdinand University. One of his examiners, the physicist Bohumil Kučera, was an expert in electrocapillarity and the examination turned into a deep discussion. Impressed, Kučera invited Heyrovský to the lab to show him his set-up: a capillary electrode from which mercury was allowed to drip as the potential was changed – the weight of each drop was plotted against potential. Kučera was having trouble making sense of the curves. Heyrovský spent two years making these excruciatingly tedious measurements, and gradually concluded that he was seeing was an electrochemical effect associated with chemistry on the surface of the droplets.
In February 1922, Heyrovský got his hands on a very sensitive mirror galvanometer. He measured the current as a function of the potential applied to the drops immersed in a molar solution of sodium chloride. As he increased the voltage Heyrovský saw a series of surges in the current. He knew immediately that he was onto something important. He called the S-shaped steps in his plots ‘waves’, which could be used to easily distinguish one ion from another. Nernst’s dream was realised. Over the next seven weeks he filled 200 pages with notes. The news started to attract students. When a young Japanese chemist, Masuzo Shikata, visited his lab in the autumn, they designed an automated instrument to speed up the experiment – polarography was born. Experiments could be done in minutes and scans could be read almost like spectra.
Polarography spread like wildfire. In 1959, Heyrovský was awarded the Nobel prize for his discovery. It would remain the electrochemical technique for choice for all manner of electroanalysis and speciation studies until the 1970s, when it was gradually superseded by cyclic voltammetry.
I’ve not seen a dropping mercury electrode in decades, but the memory of that polarography practical has never left me, along with its delicate tic, tic, tic sound.
Acknowledgment
I am grateful to Daren Caruana for inspirational lunches
I remember a practical I did as an undergraduate. I have never forgotten the strangeness of the apparatus, which included a small burette filled with mercury, droplets of which fell through a solution at regular intervals, tic, tic, tic. I had no idea of the significance of the technique – polarography – at the time, but it revolutionised electrochemistry.

The 19th century is the time when electricity shifted from a being a strange mystery to take over almost every aspect of the scientific laboratory. After Alessandro Volta’s invention of his pile it was transformed by Humphry Davy and others into a tool to tear matter apart into its components. After Michael Faraday linked electricity quantitatively with chemistry, the search turned to trying to understand how electrical currents could decompose solutions. Walther Nernst and Hermann von Helmholtz coined the term ‘electrode potential’ and developed the link between concentration and the potential itself; Nernst expressed the hope that electrochemistry would one day become as simple and useful as spectroscopy. Chemists like Max Le Blanc to begin to plot ‘polarisation’ curves to investigate the electrochemical processes – his invention of the hydrogen electrode would anchor all measurements to a common standard.
But measurements in solution remained difficult and dogged by problems of reproducibility. These issues would be swept away by a young Czech postgraduate student, Jaroslav Heyrovský. Hooked on science since childhood, he started a degree at Prague’s Charles University, where his father was rector. Heyrovský was soon so excited by the latest discoveries that he begged his father to let him study at University College, London (UCL) in the UK, where William Ramsay had been discovering the noble gases. But Heyrovský’s dream of working with Ramsay crumbled; the year he graduated, 1913, Ramsay retired.
Heyrovský was a true Anglophile; he wrote his notebooks in English to the end of his life. Awarded a demonstratorship (a fixed term lectureship) at UCL, he made plans to adopt British nationality and began to work with the newly appointed Frederick Donnan, whose recent paper on membrane potentials had cemented his academic reputation.
Donnan suggested that Heyrovský determine the reduction potential of aluminium. The oxide layer on the surface meant that its position in the ‘electropositivity’ series had been a matter of speculation and debate – some measurements placed it as high as zinc or magnesium, others suggested it was as low as iron or even lead. With the pure metal giving inconsistent results, Donnan had been using mercury capillary electrodes similar to those developed by Gabriel Lipmann . He thought that by using mercury amalgam, as Gilbert Lewis had done for the alkali metals, it would be possible to obtain a fresh surface, free of an oxide layer.
Heyrovský used a platinum–hydrogen reference electrode, using the stream of hydrogen bubbles to stir the solution and maintain an ‘inert’ atmosphere around his amalgam electrode. He established not only the reduction potential of aluminium but also gained insights into the speciation of aluminium in water by making measurements across a wide pH range.
Heyrovský was on holiday in Bohemia when the first world war broke out in 1914, and he found himself stuck in the Austro-Hungarian empire. For a few months he managed to work at the Chemical Institute in Prague, but was called up to serve in the Austro-Hungarian army. Luckily, he was appointed as a pharmacist in a hospital – a job that left him time to write up his work.
When the war ended in 1918 he submitted a thesis to Charles-Ferdinand University. One of his examiners, the physicist Bohumil Kučera, was an expert in electrocapillarity and the examination turned into a deep discussion. Impressed, Kučera invited Heyrovský to the lab to show him his set-up: a capillary electrode from which mercury was allowed to drip as the potential was changed – the weight of each drop was plotted against potential. Kučera was having trouble making sense of the curves. Heyrovský spent two years making these excruciatingly tedious measurements, and gradually concluded that he was seeing was an electrochemical effect associated with chemistry on the surface of the droplets.
In February 1922, Heyrovský got his hands on a very sensitive mirror galvanometer. He measured the current as a function of the potential applied to the drops immersed in a molar solution of sodium chloride. As he increased the voltage Heyrovský saw a series of surges in the current. He knew immediately that he was onto something important. He called the S-shaped steps in his plots ‘waves’, which could be used to easily distinguish one ion from another. Nernst’s dream was realised. Over the next seven weeks he filled 200 pages with notes. The news started to attract students. When a young Japanese chemist, Masuzo Shikata, visited his lab in the autumn, they designed an automated instrument to speed up the experiment – polarography was born. Experiments could be done in minutes and scans could be read almost like spectra.
Polarography spread like wildfire. In 1959, Heyrovský was awarded the Nobel prize for his discovery. It would remain the electrochemical technique for choice for all manner of electroanalysis and speciation studies until the 1970s, when it was gradually superseded by cyclic voltammetry.
I’ve not seen a dropping mercury electrode in decades, but the memory of that polarography practical has never left me, along with its delicate tic, tic, tic sound.
Acknowledgment
I am grateful to Daren Caruana for inspirational lunches
References
J Heyrovský, Chem. Listy, 1922, 16, 256.
J Heyrovský and M Shikata, Recl. Trav. Chim. Pays-Bas, 1925, 44, 496












No comments yet