The three winners of this year’s chemistry Nobel gave chemists the tools to make molecules into machines. Emma Stoye assembles the story
‘I’m not so sure which planet I am on!’ a somewhat dazed Ben Feringa gabbles down the phone, around 24 hours after discovering he was one of this year’s Nobel winners. Clearly, it will take a while for him to come back down to Earth. ‘I still don’t realise what happened to me,’ he continues. ‘I’m just so honoured. It’s a dream come true!’
Pursuing big dreams is something that Feringa, along with fellow laureates Fraser Stoddart and Jean-Pierre Sauvage, has always been drawn to. Through their exceptional creativity the trio, who were awarded this year’s prize ‘for the design and synthesis of molecular machines’, have given today’s chemists access to a toolbox of nanoscale shape-shifters that interact like life-sized gears, switches, pistons and propellers. One day, these may find their way into the muscles of the tiniest robots; form the basis of smart, switchable drug delivery systems; or activate new responsive materials.
David Leigh, a one-time student of Stoddart’s who now leads his own group making molecular machines at the University of Manchester, UK, sums up the excitement that buzzed through the community as soon as the prize was announced: ‘It’s absolutely fantastic for the field! It’s a great recognition of truly inspirational and ingenious inventions.’
Rings and things
‘Chemists used to look at their molecules as kind of still, motionless species… people never cared about trying to move molecular systems in a controlled fashion,’ remembers Jean-Pierre Sauvage, who spoke to Chemistry World shortly after the announcement from his home in France.
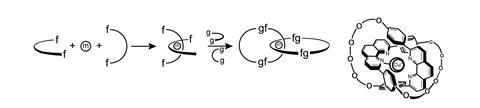
This way of thinking began to change in the early 1980s, when Sauvage, who was working at Louis Pasteur University (now part of the University of Strasbourg, where he is still an emeritus professor), uncovered a new way to make mechanically interlocked molecules, molecular rings and axles that can mimic macroscopic machines’ moving parts.
‘Sauvage was the person who, though a real stroke of genius, realised that you could make these sort of architectures through noncovalent interactions and self-assembly effects,’ says Leigh. ‘His great achievement was to take these things from being molecules that were unmakeable, to being things that chemists could actually get their hands on.’
Sauvage himself was not the first person to make an interlocked molecule. Both catenanes – two macrocyclic rings connected like chain links – and rotaxanes, which consist of a macrocycle threaded onto a dumbbell-like molecular rod with bulky ‘stopper’ regions at each end – had been successfully synthesised in the 1960s. But at that time they were virtually impossible to make in any meaningful quantities. Year after year, despite the best efforts of synthetic chemists, yields remained depressingly low.
In the early 1980s, Sauvage was a photochemist rather than a topologist. He and his colleagues were interested in artificial photosynthesis, and were searching for transition metal complexes with long-lived excited states for electron transfer. ‘We came across a copper complex that turned out to be very active in terms of photochemical properties, but was also the ideal precursor to a catenane,’ he remembers.
The complex comprised two crescent-shaped ligands entwined around a copper(i) ion. ‘We realised that [it] was very, very close – it was something like a non-cyclised catenane,’ Sauvage says. The group then discovered it could be converted into a catenane by connecting the open ends of the crescents to form rings, then removing the copper ion.1 This templating approach was much simpler than previous routes to make interlocked structures, and allowed them to be produced in much higher yields than ever before. It rescued the field of chemical topology and kick-started a whole new world of possibilities.
Today, lots of mechanically interlocked shapes are produced using the kind of interaction that Sauvage’s group pioneered. The tools he developed are now being used among diverse fields. Leigh’s group is known for using ever-more complex molecular interactions in their machines. Highlights from this year alone include a knotted structure that can catalyse reactions; a rotaxane-like ‘macromolecular wheatsheaf’ with three rods threaded through a single ring; and even a basic molecular robot with an arm capable of picking up and carrying cargo molecules.
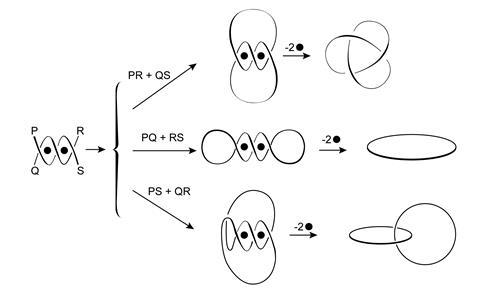
Sauvage himself quickly moved on from making simple catenanes and is known for having produced some complex shapes in the early days – including in 1989 a trefoil knot that Sauvage remembers as a ‘terrible synthesis challenge’.2 ‘People had spoken about trefoil knots but no-one had ever made one, and we thought “well, let’s try to do it!”’ he says.
From molecules to machines
Stoddart, too, is drawn to a challenge. When it comes to making molecular machines, he says, ‘you just get totally hooked!’
His interest in interlocked molecules, and later molecular machines, also developed throughout the 1980s, while he was working at Imperial Chemical Industries and the University of Sheffield in the UK.
‘The inspiration came from two sources,’ he says. ‘One was my great love of art… secondly, I was brought up on a farm near Edinburgh in the 1950s with no electricity until I was 17. I was a little bit of an engineer as a result. I learned a lot about stripping down and putting back together cars and tractors.’
Throughout the 1980s, Stoddart focused his passion for engineering at the nanoscale. ‘Stoddart used other sorts of non-covalent interactions to the ones Sauvage did – [he] realised that he could do the same things with, say, aromatic stacking interactions, where Sauvage had used metal template interactions, to make catenanes and rotaxanes,’ says Leigh, who was a PhD student in Stoddart’s Sheffield group. ‘He realised that these mechanically interlocked architectures allowed you to get large-amplitude motions of the components, and he realised that could be useful for making molecular machines.’
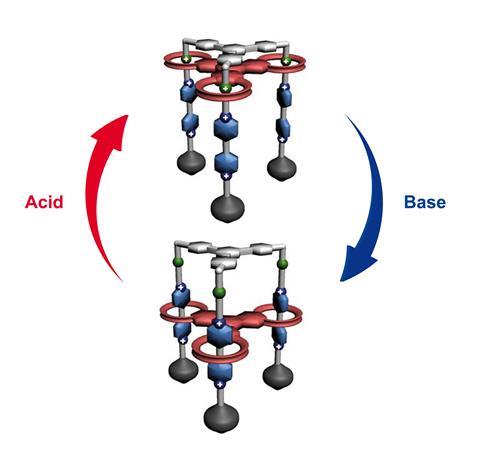
A breakthrough came in the early 1990s when Stoddart – by then based at the Unviersity of Birmingham in the UK – was able to make one of the first controllable molecular switches– a rotaxane featuring a positively charged ring that could be ‘clipped’ onto a rod bearing two electron-rich ‘stations’, then made to move between them using redox chemistry.3
‘We chose tetrathiafulvalene [for one of the stations] after a bit of exploration because this loves to go inside the [ring],’ Stoddart explains. ‘Sure enough, when we incorporated it into the rod component of the dumbbell, the [ring] really wanted to sit there 90% of the time. So now we had a switch, because we had the redox active tetrathiafulvalene unit which we could come along and take an electron or two out of.’ This makes the tetrathiafulvalene positively charged, so it repels the ring (with its four positive charges) down the rod onto the uncharged 1,5-dioxynaphthalene station.
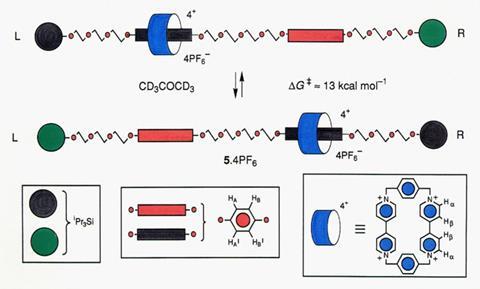
Sauvage’s group, meanwhile, was also beginning to explore ways of controlling the movement of mechanically interlocked components using electro- or photochemistry. Throughout the 1990s, both groups demonstrated various control systems. As Stoddart puts it: ‘Once we’d worked out the first switch, we were off like no-one’s business!’ Some of the most striking examples from around the turn of the millennium include Sauvage’s group’s rotaxane ‘daisy chain’– featuring two entangled rings that can be made to extend and contract,4 and Stoddart’s group’s molecular lift– a platform made of several joined-up rotaxane rings threaded onto different axles, which moves between different ‘floors’.5
Behind the wheel
Sauvage points out that a lot of the work around that time was inspired by nature’s own molecular machines: the enzymes and proteins that underpin the processes carried out by living organisms. ‘Of course we were admiring what nature can achieve,’ he says. ‘Especially kinesin, which is an enzyme that can walk on microtubules, and [the enzyme] ATP synthase which is a rotary motor,’ We thought we chemists should be able one day to make molecules reminiscent of those found in biological systems.’
Throughout the 1980s and 90s Sauvage, Stoddart and their groups continued to expand the movement and control capabilities of their interlocked molecules. This included some forms of rotation – Sauvage’s group made catenanes where one ring could be driven to rotate around the other by light or heat, for example. But it was Feringa’s group at the University of Groningen in the Netherlands that demonstrated the first fully controllable molecular motor.
Like Stoddart and Sauvage, Feringa was interested in molecular switches at the time, working to develop switches based on light-activated chiral molecules that could switch configuration back and forth to produce an on–off effect. The first true rotary motor, Feringa says, was essentially a lucky accident.
‘Ten years later, after we had developed several switches … we discovered that one of the switches not only changed chirality, but apparently moved another step forward,’ he explains.
The molecule in question consists of two ‘blades’ that isomerise around a C=C double bond, effectively rotating them 180 degrees when exposed to UV light. Feringa and his colleagues quickly realised that the molecule could be engineered to prevent this rotation form reversing direction by adding ratchet-like methyl groups to the structure. Pulses of UV make the blades rotate continuously in the same direction, like a propeller rotating around an axle.6
Once the basic mechanism was in place, the team quickly tweaked and improved the motors’ performance over the following years. ‘We made second generation motors; we made third generation motors. We increased the speed dramatically,’ says Feringa. Two years ago they unveiled a motor that could spin at 12 million revolutions per second.
The next step was to get the motors to actually do things. ‘A major breakthrough was when showed that we could move a microscopic object,’ says Feringa. ‘We put the motors in a soft liquid crystal material, put a rod on top of it which was 10,000 times the size of the motor molecule, and spin this rod. That was a distinctive moment because, for the first time, we could see movement of an object with the naked eye.’ Later came the group’s famous nano car, a central structure that could be driven along a surface using four molecular motors as wheels.7
‘This was not always easy I can tell you. In science, you get failures, a lot of failures,’ Feringa says. ‘We had some beautiful moments, but we had also disastrous moments, and this is what it’s all about. But it’s also a lot of fun and I find it a great privilege to work with all these young stars at the university.’
The collaborative spirit within his group is clearly important. Moments after his Nobel win was announced, a video of an emotional Feringa thanking the many students and co-workers gathered outside his lab started circulating online. ‘I feel very proud of everyone that worked in my team over the years.’ he says, reflecting on the many daunting challenges and breakthroughs past and present members of the group have contributed to.
Stephen Fletcher from the University of Oxford, UK, speaks of his time working in Feringa’s lab as a post-doc with fondness . ‘It was a very stimulating environment,’ he says. ‘And Ben was very good at trying to get the people that he worked with to think big and enjoy what they were doing. It’s a very good environment for scientists to be in.’
Now and next
Between them, over the last few decades, all three laureates have nurtured hundreds of students and early-career scientists. Many have taken inspiration from their mentors’ work and set up their own research groups all around the world, pushing those original ideas in new directions. The tools these early pioneers developed have laid the foundations for an area of science that is now flourishing, as molecular machines become both more complex and more controllable. Leigh says the next big step will be translating these tools into real-world applications. ‘There’s a lot of promise there, but what chemists have to do now is use molecular machines to do things that can’t be done already in a simpler way, and actually use them to try and make a kind of working molecular nanotechnology,’ he says, although he warns that trying to guess what the killer applications will be at this stage is ‘a little bit like asking stone age man, when he’s built the wheel, to predict motorways’.
Speaking by phone at the live announcement in Stockholm, Feringa used a similar analogy, saying ‘I feel a little bit like the Wright brothers who made the first flying machine 100 years ago. People were asking “why do we need a flying machine?” and now we have a Boeing 747 and an Airbus!’ Now, he says, it’s up to the next generation of supramolecular Wright brothers ‘to make the planes of the future’.
There are certainly plenty of big ideas. Like many of his contemporaries, Sauvage imagines molecular machines may well find a role in robotics or medicine. ‘Robots are becoming more and more important, and I think [one day] we will certainly have lots of robots in our everyday lives, and very tiny robots to fulfil some functions,’ he says. ‘And micro robots will need nano-muscles or nano-gears. Or of course we can imagine using molecular machines in nanomedicine to carry and deliver [drugs] to the right place.’
For Feringa, the potential to control motion also opens up tremendous possibilities in materials science: ‘When you can induce motion and make machine-type functions like rotary motion, translational motion, you can move smart materials. Materials that adapt, change their shape, and have all kinds of functions. [I think] we’ll see these kinds of smart materials appear in the next 10 years or so.’
Stoddart, who now leads a hugely productive research group at Northwestern University in the US, is also excited about electronic memory. ‘In 2007 we made a 160 kilobit memory,’ he says – referring to a device that included a collection of rotaxanes mounted between electrodes that could write memory by shuttling their rings between stations.8 ‘But that system is not robust enough – the switches would die after 100 cycles, and that’s no use,’ he says. ‘So we’re now trying to mount very similar switches inside metal-organic frameworks.’
Prizes make partnerships
While molecular machines research stands on the cusp of becoming an applied discipline, it is important to recognise the tremendous impact some of the early, fundamental work has had throughout science. It would be impossible to list all the fields that have used these tools to enhance the research – building molecular machines or interesting topologies offers unparalleled insights into how molecules work and behave.
‘I think there are tremendous opportunities if we team up with our colleagues from other disciplines,’ says Feringa. ‘We work a lot with physicists, with medics, with biologists. There’s a huge community and this has grown tremendously in the past few years because people realised that there are many possibilities.’
Of course, it is likely that the recognition from Stockholm will attract more excitement to this already burgeoning field. ‘When a Nobel prize is given, it boosts the field in a very spectacular fashion most of the time,’ Sauvage points out. This could range from attracting more funding to support basic research, to encouraging multidisciplinary or international partnerships by attracting the attention of a wider range of potential collaborators.
‘The work of these people – it’s inspirational science,’ sums up Leigh. ‘It’s inspired a lot of work in recent years and I’m sure that this prize is going to inspire the next generation of researchers to accept the challenges [ahead].’
Although nobody knows where this dynamic area of research is ultimately headed, it seems certain that the molecular wheels of creativity will keep on turning for years to come.
References
1. C O Dietrich-Buchecker, J P Sauvage and J P Kintzinger, Tetrahedron Lett., 1983, 24, 5095 (DOI: 10.1016/S0040-4039(00)94050-4)
2. C O Dietrich-Buchecker and J P Sauvage, Angew. Chem. Int. Ed., 1989, 28, 189 (DOI: 10.1002/anie.198901891)
3. P L Anelli, N Spencer and J F Stoddart, J. Am. Chem. Soc., 1991, 113, 5131 (DOI: 10.1021/ja00013a096)
4. M C Jiménez, C O Dietrich-Buchecker and J P Sauvage, Angew. Chem. Int. Ed., 2000, 39, 3284 (DOI: 10.1002/1521-3773(20000915)39:18<3284::AID-ANIE3284>3.0.CO;2-7)
5. J D Badjić et al, Science, 2004, 303, 1845 (DOI: 10.1126/science.1094791)
6. N Koumura et at, Nature, 1999, 401, 152 (DOI: 10.1038/43646)
7. T Kudernac et al, Nature, 2011, 479, 208 (DOI: 10.1038/nature10587)
8. J E Green et al, Nature, 2007, 445, 414 (DOI: 10.1038/nature05462)

![Structural Characterization of r[5]C24+](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/2/4/0/138240_CHEMPR499-10-7b.jpg)






No comments yet