Ben Valsler
This week, Katrina Krämer speaks to a researcher who is hoping to tame a dangerous reagent with salty solvents.
Katrina Krämer
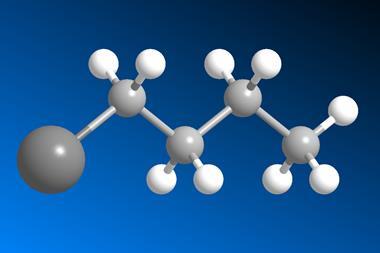
There are some chemicals you really don’t want to mess with – dioxygen difluoride, for example, which reacts violently with all sorts of compounds even at –180°C; or mercury azide, which not only detonates upon the slightest provocation but also spews out toxic mercury compounds when it explodes. Luckily, these are chemical curiosities, something researchers made once but are unlikely to ever make again (for good reason). However, other dangerous compounds are just so useful that chemists can’t keep their hands off them. One of them is tert-butyl lithium, also called t-BuLi: a staple for synthetic chemists that has a rather pyrophoric side to it.
As a main group organometallic chemist, Eva Hevia from the University of Strathclyde has not only worked with t-BuLi but also with a lot of other alkyl lithium compounds.

Eva Hevia
Organolithium compounds are widely used across the world and it’s not just in academia – in may industrial and commercial situations these reagents are essential. I believe it’s 90-95 per cent of drugs that are manufactured by pharmaceutical companies, they require, at least in one step of their synthesis, the use of these reagents. So the implications for society, and also for the economy, are vast.
Katrina Krämer
These compounds remain popular in part due to the large polarity across the lithium-carbon bond, which makes them extremely reactive.
Eva Hevia
But of course, reactivity always comes with a trade off. The trade off here is that these reagents sometimes suffer from low selectivities; sometimes they limit a lot the type of substrates that they can be compatible with. So to try to overcome these limitations, in many cases you have to use these reagents under very low temperatures, with solvents that are relatively toxic and you have always to use them in the absence of air or moisture because they decompose very quickly. And in fact, many of them are pyrophoric, so you have to be very careful how you manipulate these reagents. This can be particularly challenging when we are looking at reactions on a larger scale in an industrial scenario.
Katrina Krämer
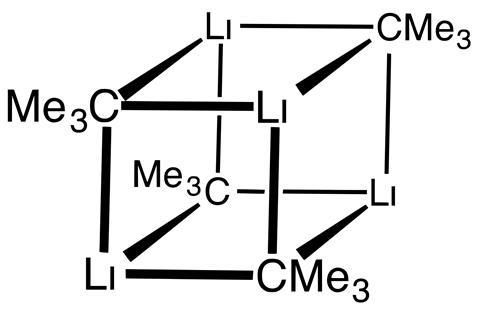
Lithium is one of those elements that really wants to get rid of one of its electrons, which it pushes towards the neighbouring carbon. But what really makes t-BuLi reactive, compared with its much friendlier linear cousin n-butyl lithium, is that the tertiary carbon doesn’t enjoy dealing with lithium’s surplus electron. Being surrounded by three methyl groups that are already mildly electron donating plus the extra electron means the central carbon has a lot of localised negative charge. Consequently, t-BuLi is highly basic and plucks protons from everything it can reach: from slightly acidic organic compounds to common solvents like tetrahydrofuran or diethyl ether, and, of course, water.
Even small amounts of water (such as water vapour in air) and oxygen are enough to produce a violent reaction. Upon contact with air, t-BuLi spontaneously bursts into bright orange flames. Since t-BuLi also reacts with most solvents, chemical companies sell it as a dilute solution in unreactive hexane or pentane, both of which are also flammable. This means if there’s an organolithium fire, there’s usually some kind of flammable solvent in the mix, too.

The tragic case of UCLA student Sheri Sangji, who died in 2009 from major burns after an experiment involving large amounts of t-BuLi went wrong, should remind every chemist that this compound needs to be treated with respect. Researchers working with t-BuLi must adhere to strict safety measures: using only small amounts and carrying out reactions under exclusion of air and moisture while wearing non-flammable clothing and having both a concerned labmate and an emergency shower nearby are the bare minimum requirements of handling t-BuLi safely.
While alkyl lithiums are powerful reagents, they are also prone to side reactions, forming useless by-products. This, on top of the safety concerns, is why chemists usually carry out reactions at –78°C and maintain an inert gas atmosphere within the reaction vessels to keep out air and moisture.
Eva Hevia
And I suppose the ultimate challenge in this type of chemistry is how we can use them in a normal atmosphere, without the presence of argon or nitrogen gas – under air – and how we can make these reagents compatible with solvents that are environmentally benign. And what we have found is that if we use deep eutectic solvents as an alternativbe media – and a deep eutectic solvent is a new type of solvent, related to ionic liquids, which are environmentally benign, made up of components that are biodegradable and biorenewable – if we use these type of solvents we can activate organolithium solvents in such a way that the reactions happen extremely quickly at room temperature. Because they are very quick, you don’t need to use an inert atmosphere, and you can make these reactions compatible with water or moisture.
Katrina Krämer
Deep eutectic solvents are mixtures of different salts that are liquid at room temperature. While most ionic compounds have a very high melting point (sodium chloride for example clocks in at just above 800 degrees), mixing the right salts in the right amounts makes a eutectic – a mixture that melts at a much lower temperature than its individual components. These rather unusual solvents are non-toxic and quite cheap – one of the compounds Hevia’s team uses is choline chloride, a chicken feed additive.
In deep eutectic solvents, organolithium reactions are much faster than in any other solvent. This would suggest that already highly reactive alkyl lithium compounds are becoming more reactive in the salty solvent mixture, which seems counterintuitive. How come Hevia’s team had no need for controlling the reaction by cooling it or conducting it under inert atmosphere?
Eva Hevia
When you work in these systems, the main enemy of your reactions is hydrolysis or decomposition of your organometallic reagent. So we activate it in a way that we can overcome this decomposition pathway. At the same time, we tune their selectivity, so we are finding in the type of reactions that we are looking at that we are getting better selectivities than when we use conventional solvents.
Katrina Krämer
Because they are made up of ions, deep eutectic solvents are very good at stabilising charged or highly polar molecules; but exactly how they work their magic isn’t clear yet. Hevia thinks that the salty solvents could also tame other more spirited organometallic reagents.
Eva Hevia
Some of our initial work has shown that we can use Grignard reagents which are also very fundamental and very important in synthesis. And some of the work that is currently undergoing in our research groups shows that these solvents offer also great potential for organo-zinc reagents which again are widely employed in many carbon-carbon bond forming processes, and they are also very reactive in the presence of air and moisture. So I think there are two ways to look at this work: first, we are developing new methods to use these reagents under environmentally benign conditions, but secondly – and this is something that I really want to emphasise – we are activating these organometallic reagents, whether this is organo-lithiium, -zinc or -magnesium, to make them work even better than when we use inert atmosphere conditions or volatile organic solvents.
Katrina Krämer
Although chemists will still need to handle t-BuLi and its brethren with care, deep eutectic solvents can help them to guide t-BuLi’s cannonball reactivity with more arrow-like precision.
Ben Valsler
That was Katrina Krämer, speaking with Eva Hevia from the University of Strathclyde about tert-butyl lithium or t-BuLi. Next week, Brian Clegg is back with a family of compounds with some concerning uses.
Brian Clegg
They are remarkably constructive – rarely directly of use themselves but each is an important contributor of building blocks to other compounds. Unfortunately, though, some of those final products can be used in chemical weapons.
Ben Valsler
Join Brian next week to find out more. Until then, let us know if there’s anything you’d like us to cover – email chemistryworld@rsc.org or tweet @chemistryworld. I’m Ben Valsler, thanks for listening.











No comments yet