Unexpected discovery enables simple conversion of waste glycerol into methanol, closing the sustainability loop for biodiesel

In the transesterification process of biodiesel production, the carbon chain of a molecule of vegetable oil is broken into three. At each break, a hydrogen atom from methanol is substituted for the link to the adjacent carbon atom. The production of biodiesel, however, leads to the formation of large quantities of crude glycerol – around 10% of the mass of biodiesel created – but is generally uneconomical to refine. Researchers are seeking ways to convert this waste product into something useful, and some efforts have focused on the dehydration reaction to acrolein – used as a herbicide and polymer precursor. This reaction is usually acid-catalysed, but researchers at Cardiff University considered that it could also be base-catalysed and investigated this using magnesium oxide. Much to their surprise, they found that the main product was not acrolein but methanol: 'We made [lead author Muhammad Haider] do an awful lot of experiments to convince us that this was real!' says principal investigator Graham Hutchings of Cardiff University.
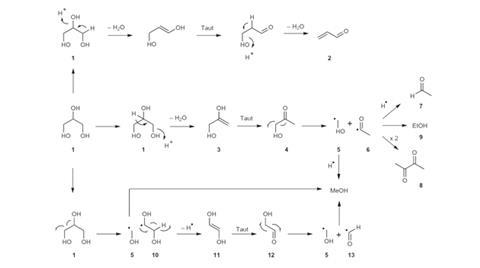
Catalysis chemist Edman Tsang of the University of Oxford describes the result as 'a bit of a shock'. The accumulation of glycerol in biodiesel production 'is an environmental problem', he says, and groups including his own are investigating various ways to solve it. 'This looks like a very simple and straightforward way.' He cautions, however, that the long-term stability of the catalyst needs investigation, and worries that the large number of different minor products could make purification of the methanol expensive.











No comments yet