Meera Senthilingam
This week, it's not just fuel that's fought over by international governments; certain pharmaceutical compounds are just as precious, as Brian Clegg explains:
Brian Clegg
Chemical compounds crop up in all sorts of places, but no one would look for one in the treaty that ended a war. Yet that is exactly where you can find the wonder drug of the early twentieth century, aspirin.
This is a compound with an ancient ancestor. It's not certain just how far back the bark of the willow tree and extracts of the plant spiraea, or meadowsweet, were used to relieve headaches, fevers and inflammation, although willow did find its way onto a list of medical supplies dating from the Sumerian Third Dynasty of Ur, around 2000BC, and was also in use for managing fevers in ancient Egypt.
Around 2500 years ago, the ancient Greek philosopher Hippocrates noted the effectiveness of powdered willow bark as a painkiller, and by the 18th century, no medic worth his salt would be without willow bark extract or meadowsweet syrup in his supplies.
Oddly, in the 18th century willow bark increased in popularity as a result of a mistake. It was recommended in a report to the Royal Society as a substitute for the much more expensive Peruvian bark, or cinchona bark, used to counter malaria. This was a case of confusion. Peruvian bark is a source of quinine, which attacks the cause of malaria, while willow bark merely suppresses the fever symptoms. Yet this error ensured that powdered willow bark went from strength to strength.
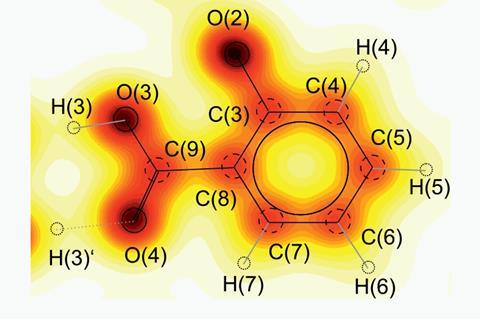
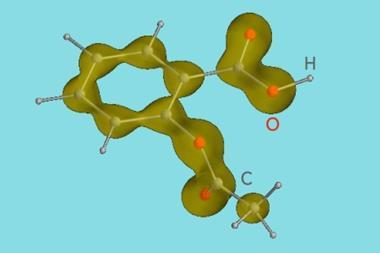
Those who were dosed with this herbal treatment often found it an unpleasant experience. The active ingredient, which we now know to be a salt of salicylic acid, does reduce pain, but at a price. Salicylic acid causes sharp stomach pains, disrupting the digestive processes and potential causing internal bleeding. This compound, which gets its name from the Latin for the willow tree, salix, is a benzene ring with two attachments: a phenolic hydroxyl, or OH group, and a carboxylic acid group.

For a long time the risk was suffered, because the drug's benefits as a painkiller and anti-inflammatory trumped the side effects. But in 1899, the German chemical giant Bayer brought out Aspirin, a trade name for a compound called acetylsalicylic acid. This was first produced by French chemist Charles Gerhardt in 1853, combining acetyl chloride with the sodium salt of salicylic acid. The process transforms salicylic acid's hydroxyl group into an ester.
The great thing about acetylsalicylic acid is that, though still capable of damaging the stomach, it is much less violent in its impact than salicylic acid, while retaining the painkilling and anti-inflammatory action. Bayer called it 'aspirin' as a contraction of the German name for the chemical, acetylspirs?ure. It joined Bayer's popular cough suppressant medicine - heroin - as a leading medical brand name. No one but Bayer could call their acetylsalicylic acid remedies aspirin.
On the 28th of June 1919, the treaty of Versailles was signed, laying out the formal reparations that would be laid on Germany at the end of the first world war. As might be expected, much of the treaty was concerned with boundaries and military restrictions. But it also had a significant financial component, and there among the huge payments and heavy industry provisions for steel and coal was the freedom to use the name 'aspirin'.
Where in Germany, Aspirin with a capital A remained a trademark of the Bayer company, as it still is today along with 80 other countries around the world, in the UK and other signature countries, the name aspirin could be freely used. This importance given to an apparently humble painkiller reflects the significance awarded to aspirin during the devastating Spanish flu pandemic that had so battered both sides towards the end of the war, where it was considered highly effective at reducing fevers.
For around 50 years, aspirin's reign was unchallenged. But by the 1970s it had been largely supplanted by the more stomach-friendly paracetamol. This is known as acetaminophen in the US, better known under trade names like Panadol (which is Bayer's brand) and Tylenol. Paracetemol would later be joined by ibuprofen and diclofenac as popular painkillers and anti-inflammatories, pushing aspirin aside. However this drop in aspirin's fortunes was countered a decade later with increasing evidence that aspirin could help prevent heart attacks and strokes.
As a pain killer and anti-inflammatory, aspirin switches off an enzyme, cyclooxygenase, which results in reduced production of a pair of lipid molecules, hormones that transmit pain messages to the brain and induce inflammation. But the new use came out of the discovery that aspirin also restricts the molecule thromboxane from making platelets clump together. Platelets are fragments of cells that circulate in the blood and contribute to the clotting mechanism. When they aggregate they can obstruct blood vessels, leading to heart attack or stroke, which is why aspirin's action is so useful. A low, long-term dose of aspirin is now regularly prescribed for those at risk.
For whole generations, aspirin was the first line of attack when suffering pain, and now it has revived its fortunes thanks to its action on platelets. Around 35,000 tonnes of aspirin are consumed each year. Few other medical compounds can boast the staying power of the humble drug that featured in the treaty of Versailles.
Meera Senthilingam
Humble, yet powerful. That was Brian Clegg with the chemistry of the sturdy, long-lived coumpound, aspirin. Now next week, ever thought about a scientific musical? Well Simon Cotton has:
Simon Cotton
There's a fortune to be made by the person who writes Chemistry - the musical.
And if that person's listening out there, I'd like to suggest two Bee Gees numbers that just ask to be part of the lyrics... Staying alive - well, that's got to be oxygen. And Tragedy is so appropriate for nylon. Let me explain.
Meera Senthilingam
To find out, you'll have to join us for next week's Chemistry in its element, with Simon Cotton. And I'm sure you will as no doubt you're dying to know why nylon is so tragic. Until then, thank you for listening. I'm Meera Senthilingam











No comments yet