Molecular movie reveals how twisting methyl disturbs aspirin electrons
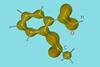
Femtosecond x-ray experiments show how small vibrations kick off electron redistribution around an entire aspirin molecule
Thousands of x-ray snapshots each lasting just a quadrillionth of a second have revealed how tiny rotations of a methyl group in aspirin drive electron relocation across the entire molecule – huge distances in molecular terms. The resulting molecular movie might help explain why aspirin’s elusive second crystal type – discovered only a decade ago – is unstable above –173°C.
