Trigold anion is first example of strange twisted aromaticity
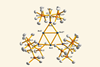
A blue tin–gold cluster is the first to show in-plane σ-Möbius aromaticity
It’s blue, contains only three gold atoms and three tin atoms, but is packed with odd aromaticity – it’s the first stable trigold anion ring that’s also the first σ-Möbius aromatic molecule. In most traditional aromatics like benzene, the orbitals that overlap and allow the electrons to freely run around the ring and are therefore responsible for aromaticity, are of the π variety. But overlapping σ orbitals can also house delocalised electrons.
