Chemists in India have published what they say is the first evidence for hydrogen bonds with tetravalent carbon atoms in proteins. Himansu Biswal’s team at the National Institute of Science Education and Research (Niser) in Bhubaneswar found 1051 such non-covalent interactions in existing Protein Data Bank (PDB) crystal structure information.
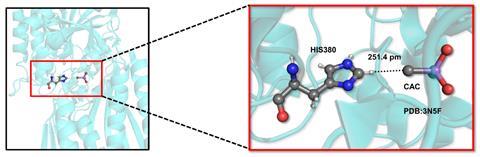
Though the strongest such interactions don’t quite match a weak conventional hydrogen bond, they can help proteins bind their substrates, Biswal tells Chemistry World. ‘If present in abundance, the cumulative effect could be large enough to control the structure and functions of the proteins,’ he says.
The discovery is surprising because the origin and nature of these hydrogen bonds are very different to conventional ones. On one side such interactions usually have a hydrogen bond donor – namely a hydrogen atom, attached to an electronegative atom like oxygen. Conventionally, hydrogen bond acceptors are also electronegative atoms, with electrons as lone pairs or in π-orbitals from multiple bonds. That’s not true for the carbon atoms that are the hydrogen bond acceptors here. The hydrogen bond donors are also often not attached to electronegative atoms.
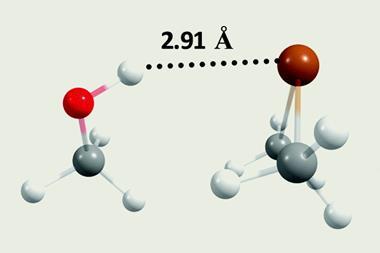
On the electronegativity scale, carbon has a value of 2.55, very similar to hydrogen at 2.2. People probably therefore thought that it couldn’t gather enough electrons to be a hydrogen bond acceptor, says team member Juhi Dutta. But in recent years, scientists have shown that carbon atoms in methane, which has neither lone pairs nor π-electrons, can be hydrogen bond acceptors. Yet no-one had looked for similar interactions in protein crystal structures, which are a rich potential source of data, thanks to the PDB.
Using home-written code, the Niser team searched the PDB for non-covalent interactions involving tetravalent carbons attached to electropositive elements like arsenic. The extra electrons arsenic contributes enable the carbon atoms to become hydrogen bond acceptors. The search used two key criteria, explains Niser’s Akshay Kumar Sahu. First, a carbon and hydrogen atom had to get closer than the sum of each of their usual van der Waals radii, which defines how near unbonded atoms can approach each other. Second, the hydrogen atom, the atom it was covalently bonded to, and the carbon atom hydrogen bond acceptor had to be aligned in nearly a straight line.
The researchers found 1051 interactions in 918 different crystals. 843 of the crystals had interactions between tetravalent carbon hydrogen bond acceptors and hydrogen bond donors where hydrogen was attached to carbon, rather than a more electronegative atom.
Marta Mosquera from the University of Alcalá, Spain says that the ‘study convincingly demonstrates the presence of H-bonds with tetravalent carbons in a complex system such as proteins’. She adds that it may encourage researchers to ‘revisit other reaction mechanisms and biological processes to explore the influence of these not-so-weak interactions’.
Biswal’s group now wants to provide experimental evidence for these hydrogen bonds using high-resolution laser spectroscopy. They also hope to find evidence that biological molecules also contain ditetrel bonds, which involve non-covalent interactions between an electron-rich carbon atom and an electron-deficient one.
References
J Dutta et al, J. Chem. Inf. Model., 2022, DOI: 10.1021/acs.jcim.2c00015













1 Reader's comment