Orbitalets – a type of localised molecular orbitals – depict chemical reactivity in large molecules in a more intuitive way than other orbital descriptions.
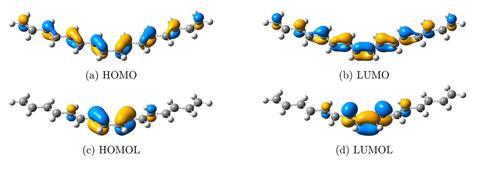
Like other frontier molecular orbitals, orbitalets express the quantum mechanical nature of electrons in molecules. They help chemists understand compounds’ reactivity and regioselectivity. But in large molecules with many delocalised electrons, the clear picture orbitals paint in smaller molecules can become blurred – it becomes hard to pinpoint the most reactive bonds and their energy. There are ways to force localisation when calculating orbitals, but some can only be applied to one type of compound while others lose important information about orbitals’ energy in the process.
Orbitalets localise even large molecules’ frontier orbitals in physical and energy spaces in a way that’s intuitive to chemists. They were developed by a team at Duke University, US, from an error-correcting method for density functional approximations.
For example, regular orbital descriptions for hexadeca-1,3,5,7,9,11,13,15-octaene make it difficult to spot the Homo/Lumo interactions typical for a Diels–Alder reaction with ethene. The compound has such a large delocalised electron cloud that all the orbitals around the reactive bond look almost the same. With orbitalets, the reactive centre can be clearly distinguished as a butadiene-like structure sitting at the chain’s centre. Orbitalets also maintain the overall energy structure that can be gained from calculating canonical molecular orbitals, though a small part of the energy information is traded for better localisation.
The Duke team also found orbitalets effective for understanding 1,3-dipolar cycloaddition reactions, intra- and intermolecular charge transfer, as well as bifurcating reactions, in which a single transition state can lead to two different products. Orbitalets should also be able to describe and predict reactivity on surfaces and interfaces, the researchers suggest.
References
J Yu et al, J. Am. Chem. Soc. Au, 2022, DOI: 10.1021/jacsau.2c00085















No comments yet