Chemists have been making artificial DNA base pairs for 20 years. Where will this race end and can anyone even see the finish line, wonders Josh Howgego
Tinkering around with the nucleotides that make up DNA; swapping around hydrogen bonding patterns, substituting Gs for Zs, and even trying to create new ones from scratch. This is what chemists like Floyd Romesberg have been doing for more than a decade.
Romesberg, of the Scripps Research Institute in California, US, says he is now close to a breakthrough that would see his synthetic base pairs being replicated in DNA alongside the familiar adenine, thymine, cytosine and guanine bases in living cells for the first time. But because progress has been incremental in this field, Romesberg was concerned people might not take much notice of his latest work. ‘I was worried people would say “Well, you’ve been making progress for a long time – why is this progress any different?”’ he admits. ‘But it’s like American football: that last yard to cross the goal line makes the difference.’
But on the playing field of xenobiology – where scientists design cellular information machinery from first principles – there are several teams in the running. Unlike Romesberg, not all of them have the goal of creating life based on unnatural DNA in their sights.
History base
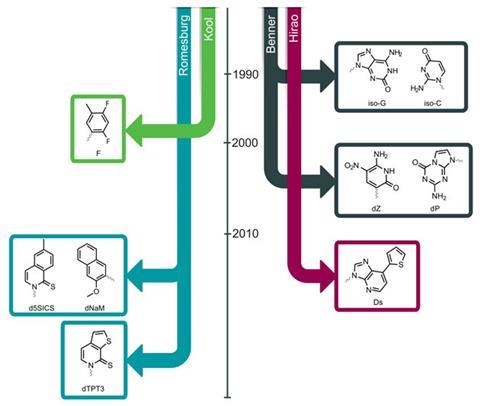
Most people credit Steven Benner with the first successful attempts to synthesise unnatural nucleotides that could be replicated in series with regular ones. Benner, based until 2005 at the University of Florida in the US, began expanding the genetic alphabet by keeping the bases’ chemistry and structure broadly the same, but varying the pattern of hydrogen bond donors. In 1990, for example, this resulted in a pair of bases he called iso-C and iso-G (for pictures of all the main structures, see the timeline below).
In the mid to late 1990s, chemist Eric Kool of Stanford University in the US extended Benner’s work by substituting a fluorine atom for one of the hydrogen atoms. The plan was to avoid some of the problems with Benner’s early bases, in which hydrogen atoms would jump around and disrupt the hydrogen-bonding pattern. But Kool soon found his fluorine-containing base, dubbed ‘F’, was awful at hydrogen bonding. Yet it was still included in replicating DNA due to its hydrophobicity. In fact, the story that emerges from the early years of chemists’ monkeying with nucleotides is that, despite its reputation for specificity, the polymer at the heart of life turns out to be rather malleable.
Romesberg took the idea that bases could partner up based on hydrophobic forces and ran with it. During the mid-2000s, his labs systematically prepared tens of candidate nucleotides and tested thousands of combinations for how well they could be included in DNA, using structure·activity relationships of the sort used in drug development – plus their own intuition – to guide their efforts.
Today there are at least three research teams working seriously on artificial bases. Benner has created the Foundation for Applied Molecular Evolution in Florida, which supports his research by synthesising and selling his bases to serve the growing demand for them in medical research. Ichiro Hirao, at Japan’s RIKEN Systems and Structural Biology Center, is also working on his own bases. Benner, Hirao and Romesberg are ‘all keeping pace with one another; they all seem to have bases which are roughly as good as one another’, says Phil Holliger, a chemical biologist at the Medical Research Council’s Laboratory of Molecular Biology in Cambridge, UK. ‘Occasionally one of them will make a leap ahead, but so far no one really seems to have really left the others behind.’
Blurred lines
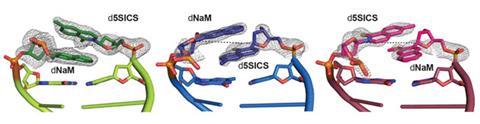
Last year though, Romesberg and his team achieved a first. Working with crystallographer Andreas Marx from the University of Konstanz, Germany, they solved the crystal structure of a DNA polymerase caught at various stages of copying their best-performing unnatural base pair, a duo of molecules Romesberg has christened d5SICS and dNaM.1
The snapshots they obtained gave Romesberg’s team a key insight. They already knew that the base pairing was driven by hydrophobic interactions between the two aromatic rings on complimentary pairs. In a free DNA duplex, these interactions resulted in the bases overlapping with each other, instead of pairing edge-to-edge as natural bases do. The crystallography revealed, however, that polymerase prompted the unnatural base pair to disengage and re-pair in an edge-to-edge manner, forming the natural base pair-like structure required for replication. This led Romesberg to think that a weaker hydrophobic force between the two bases – in effect sticking them together less tightly so they can shift between the two pairing modes more easily – might lead to more efficient replication.
Combining this insight with the knowledge they had from previous structure·activity relationships, the team then designed a slew of new molecules with smaller hydrophobic surfaces. ‘This is the opposite to what we’ve always done,’ explains Romesberg. ‘But based on the structural data we thought maybe we need to pare back the stacking ability a little.’ The results were impressive. Romesberg says one new pair, called dTPT3–dNaM, is replicated with much higher efficiency and fidelity than any of his previous efforts.2 In fact, he says, ‘with blurry vision, this is virtually as good as a natural base pair’.
It is difficult to quantify exactly how well the base pair performs in comparison with others in the literature, because such measurements depend on subtle experimental factors. For example, the type of polymerase can make a difference to the amount of mis-pairings, and the time allowed for the kinetics experiments can influence measures of how quickly bases are copied. Unnatural bases in the literature often quote impressive-sounding figures on these fronts, in part because the conditions can be chosen to suit the desired results. Yet Romesberg insists that he’s been able to use his dTPT3 molecule in a setting that underscores its qualities. His lab has been making great progress towards replicating DNA containing the pairs in living cells for the first time, he says. Though he is cagey about the details – mainly because he hopes to publish his work in the coming months · he says progress has been ‘better than, frankly, we dared to dream: this is a good base pair’.
Romesberg is now focusing his efforts on building cells that can survive with his unnatural nucleotides in their DNA. But while having unnatural nucleotides replicated in living cells is not far away, it’s unlikely that scientists will succeed in getting those cells to survive outside of the lab for long in the near future. For one thing, the unnatural nucleotides would disrupt cells’ protein coding, so life would be shortlived. Getting a cell working sustainably with xenonucleic acids in it would be a huge milestone, but it is still a long way off.
Robust concerns
Not all scientists think that deploying unnatural bases in cells is the field’s most pressing challenge. For Holliger, the point about creating new evolvable genetic languages is that it can increase the potential for storing information. And there’s much to be done on this front before life even enters the equation, he says.
For example, in order to have useful expanded genetic languages, ‘you really need to be capable of inserting the new letters you have made at any position you like, including next to each other’, says Holliger. The science is not quite there yet. For example, with Romesberg’s d5SICS–dNaM pair anything much beyond two of the unnatural bases in a row results in serious copying errors, although the new dTPT3–dNaM pair hasn’t been tested in this regard yet.
You need to be capable of inserting the new letters you have made at any position you like
Phil Holliger
Unlike Romesberg, both Benner and Hirao are most interested in deploying their new genetic letters in applied science. Specifically, as aptamers: strands of DNA that have been engineered to bind targets such as molecules, proteins or even cells. Such strands could be useful in medicine, for example as markers for disease-signifying proteins.
Yet Holliger points to several problems with this idea. ‘The reason aptamers haven’t been successful in the clinic is because their backbone is so weak,’ he says. ‘It’s degraded pretty quickly in blood serum, and so, to get around this, any aptamers that are proved to be good binders have to have a few tricks played on them to harden them up – and this can reduce their activity.’
With this in mind, Holliger’s initial approach to xenobiology has been to change the backbone of DNA and avoid altering the molecular stitches that knit the bases together. In 2012, he reported the evolution of a DNA polymerase so that it would accept and copy six new forms of DNA where the backbone sugar – normally ribose or deoxyribose in RNA and DNA – was switched for alternatives including arabinose and anhydrohexitol. He also showed these could be used to produce aptamers.3
‘We’re still working on this chemisty,’ says Holliger. ‘We’re now up to 10 different polymers, with the idea being that, eventually, when we have a larger range of these molecules, we’ll be able to tailor the backbone of our aptamers to work in any given environment.’
Applying aptamers
Holliger’s concerns notwithstanding, Benner has been making a living for several years from nanostructures built from his expanded genetic alphabet that already function in biological environments. ‘I admit it, compared to some of my colleagues, my approach isn’t so ambitious,’ says Benner, who has carried on making unnatural bases by simply swapping around hydrogen-bonding patterns. ‘But we do get results.’
For one thing, Benner’s chemistry has led to several spin-out ventures, including Firebird Biomolecular Sciences, which was founded in 2005. The company sells oligonucleotides that incorporate Benner’s unnatural bases, including the iso-G and iso-C pair, and bases called P and Z that he developed in 2006.4 Some of these products are used in diagnostic kits that monitor HIV and hepatitis C. Benner says that Holliger’s concerns about using aptamers in clinical applications are spot on, but argues that unnatural nucelotides actually show more promise than natural ones for clinically useful aptamers.
‘One reason that DNA aptamers may not always work in the body is that the strands have to fold up, relying on interactions between bases in the same strand to do so,’ says Benner. ‘The problem is that in the body, you’ve got lots of DNA floating around that can come in and disrupt those interactions. But when the folding is based on interactions between artificial components, it’s less likely there will be adventitious DNA around in biological samples that can disrupt that folding and function.’ He can also play other tricks, such as swapping the natural phosphate groups on DNA’s backbone for thiophosphates, which are harder for nuclease enzymes to degrade.
One fundamental question about aptamers is whether having unnatural bases in them would make for better binders than otherwise. It would make sense for this to be the case, since with a bolstered range of letters on offer, there would be more scope for favourable binding interactions. It was only last year that Hirao confirmed this. He created a large library of single strands of DNA where each included two or three of his unnatural Ds bases at pre-specified intervals. He then used a SELEX protocol to select strands from this library which bound one of two target proteins. The SELEX, or ‘systematic evolution of ligands by exponential enrichment’, technique is the current standard for producing aptamers. It involves mixing the library with the proteins and washing to select the best binders. These are then copied using PCR and mixed with the protein again. After seven rounds of selection Hirao identified aptamers that bound the proteins with impressive affinities. And crucially, when he synthesised versions of the aptamers where the Ds nucleotides were replaced with natural A or T those affinities dropped by a factor of 100. Hirao says he not only showed his fifth base ‘greatly augmented aptamer function’ but in applying PCR in this way ‘proved the high fidelity of our base pair’.5
Just a few months ago Benner made another advance in the same vein, using SELEX techniques to obtain the first example of aptamers containing artificial bases binding to a whole cell – a breast cancer cell line, to be precise.6
An unnatural future?
Nucleotides could get weirder still. Though natural polymerases are more forgiving than scientists might previously have thought, they still place constraints on what kind of bases they will accept. But two developments might get round this.

First, some chemists are eschewing biological machinery altogether, using modified polymerases that will accept utterly alien bases. David Perrin of the University of British Columbia in Canada, for example, has added on chains bearing a range of nitrogen-based functionalities to natural bases. Some of them even have charges, making them protein-like. Perrin only has four bases to work with at the moment, but that could increase in future. And though he has only made catalysts, not aptamers, so far, he is optimistic about the prospects for his approach, and Benner agrees.
Second, bases can be modified post-replication. For example, in the same paper that Romesberg reported his dTPT3 base, he also showed that a version of the compound with an alkyne side chain could be replicated almost as well. He illustrated his point by linking up the alkynes with the small molecule biotin, but in principle a wide variety of functional molecules could also be used, paving the way to intrinsically fluorescent DNA, for example.
After starting as a rather hopeful quest some 20 years ago, chemists have shown that making unnatural nucleotides is not only possible, but can be applied in a plethora of different ways. Romesberg may think he’s approaching his own goal line, but when it comes to xenobiology there is more than one way to score a touchdown.
Josh Howgego is a science journalist based in London, UK
References
1K Betz et al, J. Am. Chem. Soc., 2013, 135, 18637 (DOI: 10.1021/ja409609j)
2 L Li et al, J. Am. Chem. Soc., 2014, 136, 826 (DOI: 10.1021/ja408814g)
3 V Pinheiro et al, Science, 2012, 336, 341 (DOI: 10.1126/science.1217622)
4 Z Yang et al, Nucl. Acids Res., 2006, 34, 6095 (DOI: 10.1093/nar/gkl633)
5 M Kimoto et al, Nat. Biotechnol., 2013, 31, 453 (DOI: 10.1038/nbt.2556)
6 K Sefah et al, Proc. Natl. Acad. Sci.USA, 2014, 111, 1449 (DOI: 10.1073/pnas.1311778111)











No comments yet