Molecular machine generates different products from a common set of building blocks
A two-site supramolecular catalyst that can be programmed to mediate different reactions depending on its conformation has been designed by researchers in the UK.
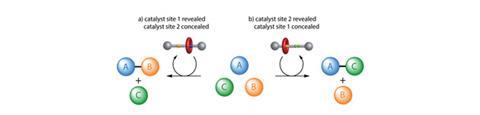
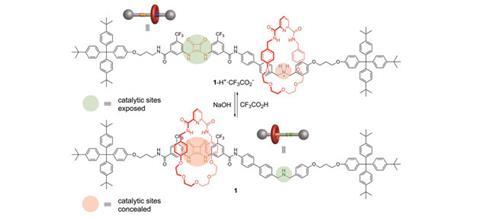
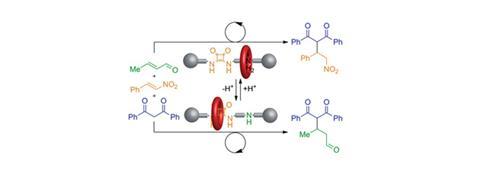
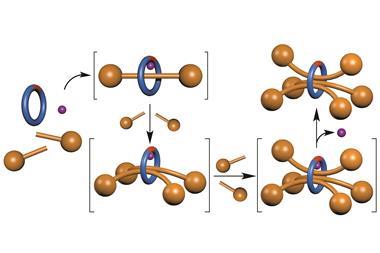
David Leigh and his team at the University of Manchester have developed a rotaxane, in which the thread contains a squaramide and a secondary amine functional group – both known organocatalysts for different reactions. The rotaxane’s macrocycle can be positioned over either catalyst, thereby blocking one’s catalytic activity whilst leaving the other site free. This means that the system can produce different products from the same starting materials according to the position of the macrocycle, which can be controlled by varying the pH of the system. This is the first example of a synthetic catalyst that can switch between two catalytically active sites.
The ability of this catalyst design to produce different reaction outcomes, with good selectivity, by targeting specific components from a common set of substrates is particularly impressive according to Stephen Loeb, an expert in mechanically interlocked molecules from the University of Windsor in Canada. ‘Combining mechanical molecular switching with catalyst selectivity is a significant step towards the goal of using integrated molecular systems to control chemical reactivity.’
Supramolecular chemist Paul Beer from the University of Oxford, UK, is similarly impressed: ‘[this work] beautifully demonstrates the proof-of-concept use of interlocked molecular catalysts to selectively react specific components of a reaction mixture. The next challenge might be to apply such a method to asymmetric catalysis.’
Ken Leung, who specialises in rotaxane catalysis at Hong Kong Baptist University, agrees that the potential applications of systems such as this could be exciting. ‘This research is garnering the interest of pharmaceutical industries and will make important contributions to our community,’ he says. ‘Leigh’s [2]rotaxane can provide insights for the design of more sophisticated smart machines for future catalyst needs, potentially obtaining a single targeted drug product or multiple products in multi-step reactions in one pot with a universal rotaxane catalyst.’
Designing more catalysts like this for more complex sequences of reactions is certainly on the cards, according to Leigh. ‘The objective is to gain control over which building blocks react together in a mixture, by switching ‘on’ and ‘off’ different catalysts in sequence, to bring about specific outcomes from sequential or cascade reactions.’
The team is also not stopping at switching the active state of their catalysts with pH control, says Leigh. ‘We’re currently concentrating on making different molecular machines that catalyze different chemical reactions and are switched by different (orthogonal) stimuli, eg, light, cation/anion binding, temperature etc. We’d like to string these processes together to bring about multiple sequential transformations in one pot.’
References
This article is open access. Download it here:










No comments yet