Some of the chemicals in everyday use cause people to suffer allergic reactions. Emma Davies scratches the surface of their mechanisms
Some of the chemicals in everyday use cause people to suffer allergic reactions. Emma Davies scratches the surface of their mechanisms

Every now and again a hairdressing horror story makes the front pages, with the headline a variation on ‘Could your hair dye kill you?’ The culprit is a chemical called p-phenylenediamine (PPD), used in dyes since the 19th century.
PPD is present in almost all oxidative (permanent) hair dyes and up to 2.5% of adults in Europe and up to 20% of hairdressers suffer from hair dye allergies. Most people who are allergic to PPD suffer from irritated skin and some develop blisters. But an unfortunate few have a severe reaction to the chemical: their faces swell dramatically and their eyes are forced shut by swollen eyelids. Last year the UK press reported that one woman had died from PPD allergy while another was left with brain damage.
The German Federal Institute for Risk Assessment (BfR) has also raised concerns over PPD’s presence in some henna hair dyes, which people may be drawn to as a more natural-seeming alternative. In a statement, the institute warns that ‘the analysed henna hair dyes that contain PPD constitute a grave health hazard and a serious risk’. Meanwhile, young people are becoming sensitised to PPD through semi-permanent tattoos, which ‘almost inject PPD into the skin’ before being slowly metabolised, says UK-based toxicologist David Basketter.
PPD is a skin allergen, also known as a skin sensitiser – a chemical that reacts with skin protein to induce a skin, or contact, allergy. Further contact with the same or similar skin-sensitising chemical can elicit allergic contact dermatitis.
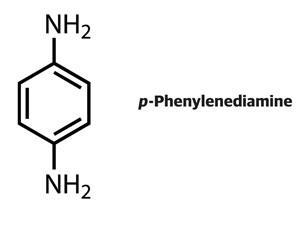
Allergic reactions to PPD are certainly not new, yet the mechanism of how the chemical acts as a sensitiser is still not fully understood. In the 1930s, Nobel laureate Karl Landsteiner and John Jacobs at the Rockefeller Institute for Medical Research, US, worked on skin sensitisation mechanisms. They proved how chemical reactivity is a key parameter for allergenic sensitisation by testing how a range of benzene derivatives reacted with aniline, chosen to mimic nucleophilic groups on skin proteins. All of the compounds that reacted with the nucleophile were found to be sensitisers. PPD was the exception in that it was unreactive yet ‘readily induces the sensitisation phenomenon’, they reported in a 1936 research paper.1 Landsteiner and Jacobs correctly asserted that the key lies in PPD’s oxidation. After oxidation, they wrote, PPD is ‘apt to enter into a firm chemical union with proteins’.
Under your skin
Today it is well known that sensitising chemicals bond covalently with proteins in the skin’s upper layer or epidermis,2 explains David Roberts, consultant and honorary researcher at Liverpool John Moores University, UK. Before full sensitisation, the body goes through a symptomless ‘induction’ phase, which can last for weeks, months, or even years, explains Basketter. Once sensitised to a particular chemical, your body will forever recognise it as an allergen. ‘It’s a lifelong problem,’ says Basketter.
The list of sensitising chemicals is long and fine fragrances, aftershaves, and deodorants are the most common sources. Up to 4% of the general population has a fragrance allergy and about a third of all allergic reactions to cosmetic products are caused by fragrance allergens. During sensitisation, dendritric cells in the skin, which alert the immune system to the presence of pathogens and other foreign materials, react to the allergen–protein complexes and migrate to local lymph nodes. There, T-cells are alerted to the presence of a foreign substance. When the skin next comes in contact with the chemical, the allergen-specific T-cells leap into action in the skin, delivering what toxicologists call an inflammatory ‘lethal hit’.
Such action can be monitored using a local lymph node assay (LLNA), which monitors the activation and proliferation of T-cells in lymph nodes during skin sensitisation in mice. Chemicals that elicit a significant increase in T-cell proliferation during an LLNA can be categorised as skin sensitisers.
Easy on the electrophiles
Most but not all skin sensitisers are electrophilic and thus drawn to nucleophilic areas on proteins such as cysteine or lysine units. Those electrophiles that are not sensitisers may simply not be reactive enough or too hydrophobic. Organic chemists will recognise the majority of electrophilic sensitisers as Michael acceptor compounds, SN2 electrophiles, Schiff base electrophiles, and acyl transfer agents.

Some sensitisers do not cause skin reactions directly but are converted in the skin into electrophilic species that do. These are known as pro-electrophiles or pro-haptens (where, roughly speaking, a hapten is a chemical that binds to a protein). Cinnamyl alcohol, found in cinnamon, is a well-known pro-hapten, as are urushiols found in poison ivy. Unfortunately, these compounds are also present in the wood lacquers used in Japan and China, which has led to some unpleasant encounters between North Americans – often sensitised to urushiols – and the lacquered toilet seats common in Asia.
Then there are the pre-haptens. These chemicals are not themselves reactive, nor converted in the skin, but they can deteriorate in the air to form sensitising chemicals, explains Roberts. They include limonene from citrus fruits and linalool from lavender, both of which oxidise to form sensitising hydroperoxides. In fact, oxidised limonene and linalool are among the most common causes for allergic contact dermatitis.
To complicate matters, some sensitisers are nucleophiles. The commonly used preservative and skin sensitiser sodium metabisulfite falls into this category. Roberts is lead author on a recent paper suggesting that sodium metabisulfite makes a nucleophilic attack on electrophilic S–S bonds in skin proteins.3
Pinning down PPD
So, which category does PPD fall into? Since it does not react with proteins but oxidises to form potent sensitisers, it could be considered a pro-electrophile. Unlike PPD-containing henna hair dyes and semi-permanent tattoos, permanent hair dyes generally also contain coupling agents such as resorcinol, which are mixed with peroxide under alkaline conditions and applied to hair. The PPD and coupling agents oxidatively join to form coloured molecules. This can prevent the formation of a potent sensitiser called Bandrowski’s base, which forms when PPD reacts with itself to form a tricyclic compound.
In any case, tests have shown that people with a PPD allergy do not react to Bandrowski’s base, says Basketter. Another obvious sensitiser is an oxidation product of PPD called benzoquinone, yet PPD-sensitive people do not react to this either. Fingers are currently pointing at a diimine oxidative intermediate, which can act as an electrophile. Some, including Roberts, suspect that the mechanism may be more complex, possibly involving free-radical binding. When PPD oxidises to lose an electron it can give a radical cation which could bind to skin proteins.
Also of concern are substances closely related to PPD, such as the hair dye toluene-2,5-diamine sulfate, once lauded by industry as the ‘safer alternative to PPD’, says Basketter. ‘The problem turned out to be that it was only slightly less allergenic than PPD and we needed to use a bit more of it to get the same effect so we ended up with the same problem,’ he says. What’s more, people sensitised to PPD are highly likely to be sensitised to toluenediamine, thanks to the chemical similarity.
Beyond the chemistry of protein binding, the exact biological mechanisms of sensitisation matter little to regulators. ‘The only thing that really matters is whether a chemical reacts with a protein, how reactive it is, and what the [chemical] mechanism is,’ says Roberts.
Allergen alert
In 1999 the EU’s Scientific Committee on Cosmetic and Non-Food Products (SCCNFP) identified 26 key fragrance allergens which now have to be clearly listed on packaging if they exceed a concentration of 0.001% in leave-on products and 0.01% in rinse-off products, under the Cosmetics Directive. The EU’s Scientific Committee on Consumer Safety (SCCS) would now like to see this list of contact allergens lengthened to include more than 80 fragrance substances;4 not that big a number when you consider that a perfume can contain over 300 basic components selected from over 2500 compounds.

Included in the original allergen list are limonene, oak moss, and eugenol – a pro-hapten present in cloves and other spices. The allergenic hydroperoxides formed by limonene oxidation have been suggested to cause sensitisation by reacting with proteins by a free radical mechanism, explains Roberts. He tells of Alpine skiers becoming sensitised to limonene when refreshing their thirst by biting into orange segments, exposing themselves to the peroxides. ‘Once you have been sensitised by eating oranges in the Alps you may react if you experience limonene, or an oxidised version of it, in perfume,’ he adds.
Meanwhile, oak moss, a type of lichen commercially harvested for its sharp, woody, fragrant compounds, is known to sensitise skin through its main allergenic constituents chloroatranol and atranol. ‘We know that atranol and chloroatranol are very, very powerful sensitisers in humans,’ says Basketter. The SCCS would now like to see the chemicals banned from use in cosmetic products.
The Cosmetics Directive bans animal tests on cosmetic products and ingredients. This includes a ban on sales of products tested on animals with exemptions for repeat-dose toxicity tests, including those for skin sensitisation and carcinogenicity. These exemptions are scheduled to be lifted in March 2013 but some NGOs are worried that the EU may delay the deadline. The European Cosmetics Association is reported to have spoken out against the 2013 total ban, asserting that non-animal tests for the repeat-dose tests are not yet ready. Indeed, a report by the European Centre for the Validation of Alternative Methods (ECVAM) estimates that it will take up to nine years to have skin sensitisation tests that are true replacements for animal experiments. Yet alternatives to animal tests are gaining recognition across the globe. For example, in February 2012, China’s State Food and Drug Administration released a draft proposal approving the use of alternatives to animal testing for cosmetics.
‘What industry would like to have is one universal set of tests or methods that they could use,’ says Terry Schultz, a skin sensitisation expert from the Department of Comparative Medicine at the University of Tennessee, US.
Alternative approach
As it stands, LLNA is the most useful tool for identifying and characterising skin-sensitising substances, and as yet is unmatched by in vitro techniques. ‘The main challenge is to address the required level of integration of the molecular and cellular processes that are underlying skin sensitisation,’ according to a recent review paper from a BfR contact dermatitis workshop in Germany.5 Several separate in vitro assays would be needed to come close to matching the LLNA.
Combining molecular structure information with information on physical and chemical properties and test data allows chemicals to be grouped together. The potential toxicity or sensitisation potency of an untested chemical can then be inferred from a set of similar chemicals within the same category in a structure and activity approach known as ‘read-across’. Quantitative structure–activity relationships (QSARs) are commonly used to evaluate the sensitising potential of allergens based on parameters such as chemical and thermodynamic constants and reactivity. Predictions work well for molecules sharing a reaction mechanism yet can be less successful for substances that can react in more than one way. For example, a,ß-unsaturated aldehydes can either form Schiff bases with nuclephilic proteins or undergo a Michael addition reaction, giving very different QSAR predictions.
Computer simulations or chemical reactivity tests can sometimes work perfectly to predict sensitisation yet there is always the possibility of false predictions, says Schultz. ‘We can do a very good job of predicting chemicals that are extreme or strong sensitisers and so you do not see those type of chemicals in personal care products,’ says Schultz. ‘But we have difficulty in separating the weak sensitisers from the non-sensitisers,’ he adds.
It’s a lot easier to design chemicals to be sensitisers than to design them to be non-sensitisers
Schultz is trying to differentiate between weak sensitisers and non-sensitisers by studying the kinetics of assays with the peptide glutathione (GSH), a nucleophile found in cells. Test chemicals are added to GSH and the amount of free thiol then measured using a spectrophotometer to estimate how strongly the chemical has reacted with the peptide. ‘The GSH reactivity assay gives you a potency answer, not a yes/no and that is what is so important,’ says Schultz.
Schultz is using his team’s GSH data for read-across to predict a chemical’s skin sensitisation potential. ‘We are trying to cover a structural space in one dimension and a potency space in a second dimension,’ he says. ‘The problem comes when a new chemical is so exotic that you haven’t tested anything similar. In that situation you would have to go back and expand your GSH chemical space,’ he adds.
Read-across is also being championed by an EU project funded by the European Commission and industry body Cosmetics Europe called Seurat-1: Safety evaluation ultimately replacing animal testing. Seurat-1’s goal is to pave the way to replacing in vivo repeat-dose toxicity testing. Mark Cronin, from Liverpool John Moores University, is coordinating the Cosmos project – integrated in-silico models for the prediction of human repeat-dose toxicity of cosmetics to optimise safety – in the Seurat-1 cluster. Cosmos uses chemical grouping to establish patterns within toxicology data.
Cronin is part of a team building a vast Cosmos database linking chemical structures to organ toxicity data, as a resource for building computational models for predicting safety thresholds for groups of chemicals. Such models should be able to predict levels of cosmetics ingredients reaching target organs. ‘We’re collecting historical data, mining data from industry and from regulatory submissions,’ says Cronin. The Cosmos database is still growing and is currently based on over 44,000 chemical structures, of which at least 7,000 are used in cosmetic formulations, he adds. ‘For these 44,000 we currently have repeat-dose toxicity data for over 800 compounds and skin permeability data [how much a chemical passes through the skin] for over 400 compounds.’ The plan is to make the database publicly available at some point in the near future.
Structure–activity relationships are increasingly used to avoid undesirable compounds in new formulations. But avoiding common problematic groups such as aldehydes, ketones, quinines and hydrazines is not easy. ‘Trying to design out sensitisation is complex – you find that you’re not left with much. It’s more about trying to estimate how potent these things are and what the safe levels are,’ says Roberts. ‘It’s a lot easier to design chemicals to be sensitisers than to design them to be non-sensitisers.’
As the cosmetics industry works to create new wonder ingredients with limited potential for sensitisation, PPD still remains a firm favourite and shows no sign of slipping from favour. Meanwhile, scientists continue to scratch their heads over exactly how PPD sensitises.
Emma Davies is a freelance science writer based in Bishop’s Stortford, UK
References
1 K Landsteiner and J Jacobs, J. Exp. Med., 1936, 64, 643 (DOI: 10.1084/jem.61.5.643)
2 H Alenius et al, Drug Discovery Today, 2008, 5, 211 (DOI: 10.1016/j.ddmec.2008.06.001)
3 D W Roberts et al, Contact Dermatitis, 66, 123 (DOI: 10.1111/j.1600-0536.2011.02038.x)
4 SCCS opinions on perfumes http://bit.ly/QHWWRq
5 M Peiser et al, Cell. Mol. Life Sci., 2012, 69, 763 (DOI: 10.1007/s00018-011-0846-8)












No comments yet