Hayley Birch discovers how researchers are using proteins from viruses to create new antimicrobial drugs
During the 2008 war between Russia and Georgia, obscure therapies were used to treat bacterial infections acquired on the battlefield. Obscure, that is, if you don’t know much about phage. Phage, or bacteriophage, have been described as the ‘dark matter’ of the biosphere.1 There are an estimated 1031 of them on the planet, lurking as viruses within the cells of their bacterial hosts. Just as viruses take over human cells, phage take over those of bacteria, eventually exploding their hosts from the inside to release their microscopic progeny. It’s this ability to blow up bacterial cells – using bacteria-bursting proteins – that makes them of interest to the military for treating sick soldiers, as well as anyone looking for new ways to treat bacterial infections without relying on failing antibiotics.
Today, one New York-based company, ContraFect, is gunning to get a phage protein approved as a treatment for bacterial infections. Their drug, CF-301, is an enzyme that can kill methicillin-resistant Staphylococcus aureus (MRSA) in mice and they hope it will do the same in humans. Raymond Schuch, vice president of research at ContraFect, explains that the enzyme was discovered in 2008, around the same time that Russia and Georgia were at war. But the idea of using these types of enzymes to kill bacteria has been around a lot longer.
From humble beginnings
Alexander Fleming initially went down a similar route before discovering penicillin. In 1921, labouring over petri dishes at St. Mary’s Hospital in London, UK, while in the clutches of a bad cold, he wiped his own nasal mucus across an agar plate, just to see what would happen. A few weeks later he noticed something in the secretions was killing bacteria. It was lysozyme, a naturally-produced antimicrobial substance found in – let’s be clear – snot. ‘It wasn’t from bacteriophage, but it was the same class of enzyme and it degrades the cell of target bacteria,’ says Schuch.
Phages act very differently to antibiotics
While the enzyme idea didn’t take off until much later, the first human trial of a phage-based therapy had already taken place. A couple of years earlier, French-Canadian self-taught microbiologist Félix d’Hérelle had extracted whole phage from the stools of soldiers and used them to treat dysentery in children, with some success. After that, and throughout much of the twentieth century, Poland, Georgia and Russia used phage cocktails to treat bacterial infections. During the 1930s, the Eliava Institute in Tbilisi, Georgia, was shipping phage to the Soviet military by the ton.
Fleming’s research, of course, changed the course of history when he found penicillin on one his plates, signalling the dawning of the antibiotic era. But Polish research from the 1980s suggests that, even then, phage therapies would occasionally come to the rescue in chronic infections where antibiotics couldn’t do the job. In the case of these whole phage therapies, the exact molecules at work may not have mattered, but with the advent of modern biotechnology came the possibility of isolating, purifying and synthesising phage-derived molecules in large quantities to create drugs like CF-301.
ContraFect’s phage-derived molecule is an endolysin or lysin, a type of bacteria-bursting enzyme that is the focus of much of the current research on phage proteins. In nature, phage deploy lysins from the inside, using them to break down the peptidoglycan polymer in the tough cell walls of Gram-positive bacteria and causing them to spew their contents – including new, infectious virus particles made by the phage. Schuch’s team at Rockefeller University spotted lysin-like genetic signatures in the genome of a pig pathogen, Streptococcus suis. Because phage force their DNA into their hosts’ genomes in order to gain control, the code was just ‘sitting there’, as Schuch recalls. ‘We got that sequence, we cloned it, we expressed it and we demonstrated that it was active against strep, which was nice, but as a side benefit it was also active against staph [Staphylococcus aureus].’ According to Schuch, phage lysins for killing staph had proven difficult to produce in large quantities until then. They’d gone through hundreds of potential molecules but had finally got lucky.
A 2014 study2 shows the cloned enzyme – expressed and produced in Escherichia coli – kills 250 different strains of S. aureus including 120 methicillin-resistant strains, and does it much faster than antibiotics. It also takes out MRSA in mice with staph bacteria in their blood. Human trials for this condition are due to start this year, following completion of phase I trials in healthy volunteers. Meanwhile, another anti-staph protein, from phage K which infects a range of Staphylococcus bacteria, is in clinical trials for use in nasal treatments with California-based biotech company GangaGen.
Lysins to kill
So what it is about lysins that give them such great potential as alternatives to antibiotics? Their main advantage is their mode of action, says Dwayne Roach, a researcher at Institut Pasteur in Paris, France, who recently wrote a review on phage proteins. ‘The driver to be looking at proteins that come from phage is that they do not rely on any modes of action that antibiotics use right now. They act very differently to antibiotics, which is very attractive because then they’re not prone to the same resistance mechanisms that are already out there for bacteria to use.’
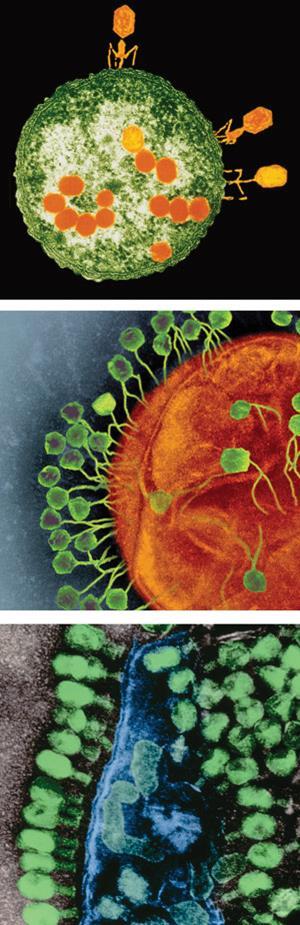
One reason why bacteria might be less likely to develop resistance to phage proteins is that, in therapeutic approaches, they’re used in a different way to the way phage use them. Instead of bursting the bacterial cell wall from the inside, the lysins being tested as drugs are applied to the outside of cells. Roach thinks this ‘unnatural’ form of attack is so foreign to the bacteria that they’re unable to mount a defence against it. By contrast, most antibiotics are molecules derived from those evolved by bacteria to fend off their competitors and thus work in ways that are fairly recognisable to them – ways they can evolve to evade. The other reason, says Roach, is simply that ‘phages are very good at being predators to bacteria’, so they’ve got some pretty unassailable methods for doing that.
Indeed, results from Schuch’s lab suggest that bacteria growing in the presence of the antibiotic daptomycin develop resistance to it at least 32 times faster than those growing in the presence of CF-301 develop resistance to the drug. In an older study on a lysin that kills the anthrax bacterium,3 Schuch and his coworkers couldn’t produce any resistant bacteria, even when they exposed their cultures to a chemical known to bring about genetic mutations. Yet in the presence of the same chemical mutagen, streptomycin resistance increased 10,000-fold. Of course, this doesn’t mean bacteria will never develop resistance to lysin-based drugs, or any other phage proteins, but it seems we might have more leeway with phage products than we do with antibiotics.
Structure solution
There are some tricky problems still to overcome though. For one thing, endolysin structures are hard to determine, particularly in combination with their peptidoglycan targets within bacterial cell walls. There are only a few endolysins that have been co-crystallised with their targets, or at least analogues of them. If you can do that, explains Mark van Raaij, a crystallographer at the Spanish National Biotechnology Centre in Madrid, you can see in atomic detail how the protein is recognising and binding to the bacterium. ‘If you know all that detailed information then you could perhaps think of doing protein engineering to adapt it to other peptidoglycans and even other bacterial strains or other bacterial species,’ he says. ‘That’s something we’re interested in.’
Endolysins are difficult to crystallise at all, according to Schuch, because of their modular structure. They’re made up of different domains joined by flexible linkers – it’s the flexibility that causes the difficulty, because it prevents stable crystals forming. Van Raiij agrees it’s problematic for crystallisation purposes, especially as it’s not always obvious which are the flexible bits, but not particularly more problematic than for proteins in general. The way crystallographers get around it is by choosing one domain, crystallising it on its own, and then repeating the process for another domain before putting the pieces back together and having a stab at predicting what the whole molecule actually looks like.
This was the approach taken for CF-301, while in 2014 Van Raaij’s team crystallised4 a portion of another lysin that attacks MRSA – a portion belonging to phage K. Van Raaij is collaborating with Aidan Coffey and his group at the Cork Institute of Technology in Ireland, who are trying to develop an antimicrobial based on the molecule. The domain they crystallised is called the CHAPK domain, equivalent to part of GangaGen’s anti-staph drug. It’s known to cut peptidoglycan between two very specific amino acids. Based on its structure and how other endolysins are thought to work, they’ve been able to make an educated guess as to a reaction mechanism for the cutting process, involving a triad of amino acids within the active site of the enzyme. But to really know what’s going on, they need to co-crystallise CHAPK with analogues of its substrate. ‘We have been trying a lot and haven’t succeeded yet,’ says Van Raaij. If they do succeed, the next task could be deciding how to go about engineering the protein.
Designed different
Rob Lavigne’s group has already started protein engineering in lysins at the University of Leuven in Belgium. His research focuses on surmounting perhaps the biggest hurdle to using lysins as broad-acting antimicrobials: in nature, lysins only work against Gram-positive bacteria, and they are only half of the problem. Many hard-to-treat bacteria – like resistant strains of the bacterium that causes gonorrhoea – are Gram-negative bacteria. In September last year, for instance, Public Health England announced that it had detected 15 cases of highly drug-resistant gonorrhoea that did not respond to one of the standard treatments recommended in Europe, the macrolide antibiotic azithromycin. The drug is usually combined with a cephalosporin called ceftriaxon. Unfortunately, cephalosporin-resistant strains have already been detected in other European countries.
But why don’t lysins work against Gram-negative bacteria? Because in these bacteria, the peptidoglycan cell wall that the molecules are so adept at breaking down in Gram-positives is not accessible to them. It’s surrounded by a further layer, an outer lipid membrane. Getting through the outer layer therefore requires either a different type of molecule altogether, or guiding a lysin through to the inner layer first. Lavigne’s team has gone for the latter approach. ‘We have designed a new strategy, which is puncturing the outer membrane, allowing the endolysin to go through and destroy the peptidoglycan layer,’ he explains.
His strategy is based on ‘designer’ endolysins, dubbed artilysins, which combine ordinary lysins with antimicrobial peptides capable of breaching the outer membranes of Gram-negative cells. These antimicrobial peptides could come from any source. For example, in the Art-175 molecule Lavigne’s team has designed to kill Pseudomonas aeruginosa, the lysin is coupled to a white blood cell protein from sheep. Drug-resistant P. aeruginosa is of major concern in hospital infections and in chronic lung infections in cystic fibrosis patients. Initial results from the test-tube suggest Art-175 kills all strains including multidrug-resistant ones,5 with time-lapse microscopy showing the bacterial cells blowing up within minutes.
Working in partnership with proteinresearch company Lysando, which licenses artilysins, the Belgian team hopes to tackle other drug-resistant pathogens too. ‘In theory, there is no limitation to which Gram-negative bacteria you could target,’ says Lavigne. ‘The artilysins are a modular approach and by playing around with these modules you can alter specificity and activity.’ One of the key benefits of the lysins, though, is their narrow specificity, which keeps them from harming the commensal or ‘good’ bacteria, for instance, on the skin or in the gut. So altering them requires a careful balance.
A realistic future
Acknowledging that the Gram-negatives are a significant threat, Schuch and ContraFect are also in the business of adapting lysins to take on these more challenging targets. Schuch is well-acquainted with the artilysins but says his own team has other modifications in mind, ‘which will hopefully take this a step further’. What’s more, both teams believe their phage-based therapies can address chronic infections caused by the thick plaques of bacteria known as biofilms. These bacterial collectives often survive antibiotic treatment despite apparent susceptibility of the individual cells.
However, like others in the field – Lavigne included – Schuch is cautious of making big claims about lysins. He prefers to think of them not as replacements for antibiotics but rather as alternatives, with the potential to treat many of the infections that antibiotics are weakening against. ‘From the very beginning, the idea was that these were going to enter into the suite of chemical entities for treating bacterial infections, so to think they’re going to replace antibiotics completely, that’s probably unrealistic.’
The US Food and Drug Administration (FDA) has put CF-301 on a Fast Track scheme to approval, meaning if all goes well it will become a first-in-class antimicrobial, initially for use in life-threatening cases in combination with antibiotics. Roach says others in the field are watching expectantly to see what will happen before piling in. ‘With endolysins, we’re right on the cusp of people jumping on board,’ he says. ‘ContraFect is one of those companies that has started out to try to be the first, but everybody else wants to be second – they’re standing in line saying “Now it’s going to happen and we’re going to stand behind it”.’
Meanwhile, a major European Commission-funded trial of old-fashioned phage cocktails is underway in burns units across France, Belgium and Switzerland. It seems phage, the ‘dark matter’ of the biosphere, are finally finding the limelight.
Hayley Birch is a science writer based in Bristol, UK
References
1M L Pedulla et al, Cell, 2003, 113, 171 (DOI: 10.1016/s0092-8674(03)00233-2)
2R Schuch et al, J. Infect. Dis., 2014, 209, 1469 (DOI: 10.1093/infdis/jit637)
3R Schuch, D Nelson and V A Fischetti, Nature, 2002, 418, 884 (DOI: 10.1038/nature01026)
4M Sanz-Gaitero et al, Virology, 2014, 11, 133 (DOI: 10.1186/1743-422X-11-133)
5Y Briers et al, Antimicrob. Agents Chemother., 2014, 58, 3774 (DOI: 10.1128/aac.02668-14)











No comments yet