Bizarre and exciting findings are emerging at high pressures even as researchers struggle to explain them, finds Andy Extance
Sodium’s soft shininess is a familiar fixture in chemistry – but by merely twisting a few screws, the alkali metal is transformed to transparency. Despite apparently happening easily, this surprising metamorphosis results from almost incomprehensibly high pressures – around 190 gigapascals (GPa), 1.9 million times the standard pressure exerted by Earth’s atmosphere.1 The screws are part of a diamond anvil cell (DAC) that concentrates force between the micron-size tips of two diamonds. The latest DACs can reach pressures beyond 1000GPa.
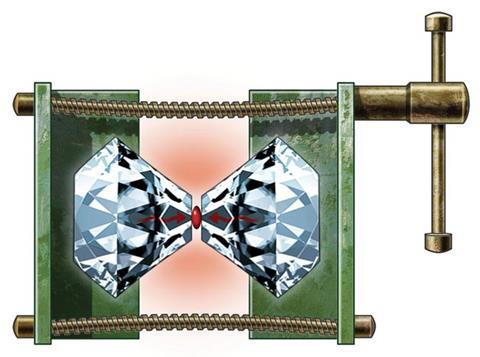
The altered appearance of see-through sodium crystals signifies pressure-driven changes happening at yet smaller scales, as electrons squeeze into ever more constrained spaces. In this way, DACs reveal puzzling phenomena. For example, at high pressures, electrons in sodium separate themselves from atomic nuclei, pairing up and huddling in spaces in between. And this strange form of sodium is an insulator, in contrast to the perception that, at high enough pressures, all materials become metallic conductors. The way that pressure changes the most stable atomic arrangements similarly forces crystals of ionic compounds into adopting odd formulae.
Most bemusing of all, existing scientific models are often bad at predicting what will happen at extreme pressures. That is a major failing, according to Artem Oganov of Stony Brook University in New York, US, who also has an appointment at the Skolkovo Institute of Science and Technology in Moscow, Russia. ‘I don’t think we truly understand chemistry if we don’t understand the behaviour of the elements under pressure,’ he says. ‘We’ve opened the doors to places where the ignorance of human beings shines.’ Oganov and other scientists are therefore trying to untangle this unpredictability, in the hope of reliably exploiting high pressures in contexts ranging from chemical synthesis to superconducting materials.
The problems originate in fundamental thermodynamics, specifically in Gibbs free energy, which reaches a minimum when a chemical system is stable. At the heart of the Gibbs free energy equation is the pV term that multiplies pressure and volume together. When pressures reach 100GPa, the pV term adds enough energy to outweigh even the strongest chemical bonds in most materials, Oganov says. ‘We can expect chemical bonding to be modified in a major way,’ he adds. Standard thermodynamic thinking would predict crystals to adopt the most tightly packed structures to reduce the pV term by minimizing volume. ‘One could expect regular close packings, but very often what we see is the opposite – dense, but irregularly packed structures,’ Oganov says. ‘The changes are hard to anticipate.’
The core issue
Nevertheless, Oganov has developed evolutionary algorithms to hunt through chemical and structural space.2 The algorithms try various structures, keeping the most stable, adapting more structures from those, and repeating the process until reaching the best solution. Such efforts helped Oganov and colleagues predict that sodium would become an insulator. But the task can be daunting with, for example, sodium chloride crystals potentially adopting an almost infinite number of ratios of sodium to chlorine ions. To make predictions relevant to complex problems efficiently, Oganov uses ‘a combination of our powerful global optimisation search algorithm and quantum mechanical calculations of free energies’, he says.
This approach suggested that an NaCl3 structure with two phases, one featuring groups of three chlorine atoms that have a single negative charge, could exist at high pressures. Likewise, Oganov’s team predicted that Na3 Cl, which has standard NaCl layers interleaved with layers of pure sodium, would also become stable.3 Both were later confirmed in DAC experiments, but other structures have evaded such calculations. ‘We predicted that above 80GPa xenon should form stable compounds with oxygen, such as XeO, XeO2 and XeO3,’ Oganov says.4 But they missed stable compounds later identified by other researchers. ‘We thought the inner core electrons of xenon didn’t play much of a role. It turns out that they do. This came as a surprise.’
Full inner electron shells, which chemists typically ignore in favour of valence-shell bonding electrons, can also help explain sodium’s transparent state. The see-through high-pressure structure is an electride – a crystal containing positively charged sodium nuclei and negatively charged electrons alongside. Why this arrangement forms under pressure was uncertain, but Roald Hoffmann from Cornell University in Ithaca, US, and Mao-sheng Miao from California State University in the US have been investigating.
In particular, Miao and Hoffmann studied the quantum nature of the isolated electrons, which they dubbed ‘interstitial quasiatoms’ (ISQs).5 Even without nuclei, the ISQ electrons can occupy quantised energy levels, just like the electrons in atoms occupy orbitals. And at high pressures, their free energy rises less than that of conventional sodium valence electrons. ‘When you compress, there is pressure on the valence electrons as they are forced closer to the core levels,’ explains Hoffmann. The lower filled shells occupy an incompressible volume, he adds, which in a sodium atom shrinks the volume available to the single valence electron as pressure increases. ‘Therefore it jumps into the interstitial space,’ Hoffmann says. Single-electron ISQs can even bond with each other to form quasimolecules, Miao and Hoffmann suggest.
The turn of the screw
Meanwhile, DAC’s practical capabilities are expanding to reach ever-greater pressures. At one level, the procedure is simple: it is just turning the screws, explains Mikhail Eremets from the Max Planck Institute for Chemistry in Mainz, Germany, whose team first photographed transparent sodium. In this way, Eremets can apply a tonne of force (around 10kN) to his tiny DAC, which he says reaches 300GPa ‘easily’. Getting to this stage has taken what Eremets calls a ‘silent revolution’, with DACs’ pressure capabilities gradually growing by more than an order of magnitude over 50 years. ‘The geometry has been optimised, the size of the sample has got ever smaller, and the diamond should be perfect,’ Eremets explains.
[[High-pressure experiments are difficult. It’s not your typical laboratory setup of a solid-state chemist]] Usually any crystals studied in DACs must be protected against being destroyed by the stress they experience, adds Natalia Dubrovinskaia from the University of Bayreuth, Germany. ‘People load neon and helium to create a very soft pressure medium in the cell,’ she explains. All materials solidify at around 12–15GPa, but neon and helium stay very soft under pressure, and stop crystals being destroyed – although they bring extra complications. ‘The pressure medium can interact with the materials and form unexpected compounds,’ Dubrovinskaia says.
Eremets and many other scientists have used DACs to pursue an 80-year old prediction that at high enough pressures hydrogen will behave as a metal. That prize became especially valuable when 30 years later more sophisticated calculations suggested that metallic hydrogen should need less cooling than other materials to become a superconductor. It might even be a superconductor at room temperature.
The trouble is, the original prediction only said hydrogen would become a metal at pressures higher than 25GPa – not how much higher. At around 260GPa, Eremets’ team has produced solid hydrogen that’s as conductive as a metal might be.6 ‘We didn’t claim strictly that we observed metallic hydrogen, because it should be proved not only from electrical measurements but also other measurements,’ Eremets concedes. ‘It’s still not a solved problem.’
Scientists also predicted many other hydrogen-rich materials might be high-temperature, high-pressure superconductors, yet hadn’t delivered practical results – until last year. Then, Eremets and his colleagues published their studies7 on hydrogen sulfide, H2 S. Above 90GPa, it separates into phases of pure sulfur and H3S, the latter making the material superconducting above 203K. The previous highest temperature at which superconductivity occurred was 164K at high pressures in a mercury-containing copper oxide material.
The downside is that the superconductivity disappears when pressure is released. But Eremets believes the hydrogen sulfide results could help chemists find substantial superconductivity at ambient pressure and even higher temperatures. ‘Very likely, it could be materials with high levels of hydrogen or other light elements, and importantly with strong covalent bonding,’ he says.
Yet these results have been controversial because other researchers struggled to replicate them until May 2016, when Japanese researchers were able to get x-ray diffraction measurements on the H3S superconducting phase, with help from Eremets and his colleagues.8 Compared with simpler replication of ceramic superconductors made in common furnaces, this situation highlights the larger problem of bringing such findings to the wider world. ‘High-pressure experiments are difficult,’ stresses Hoffmann. ‘It’s not your typical laboratory setup of a solid-state chemist.’
Coming back from the extremes
Dubrovinskaia is exploring still more extreme conditions, having helped design the highest pressure DACs existing today, which her team call double-stage DACs.9 The first stage comprises two conventional DAC diamonds. The second is a pair of 20µm diameter nanocrystalline diamond hemispheres that the Bayreuth scientists produce themselves, one mounted on each of the first stage diamonds. Among the studies this setup has enabled is squashing otherwise extremely incompressible osmium to 750GPa.10 Only small changes in osmium’s hexagonal close-packed crystal structure resulted, which might at first seem underwhelming. However, they have been interpreted as another instance where pressure brings core electrons into play, this time with them participating in chemical bonding.
Lower pressure experiments have also confirmed predictions of interesting behaviour, with Dubrovinskaia and colleagues producing a new superconductor ‘from scratch’. In 2010 scientists in the UK and Germany calculated that iron tetraboride, which at that point had never been made, should be a superconductor at 15–20K.11 In 2013, Dubrovinskaia published details of making it by heating iron–boron mixtures above 1500K and 8GPa. They found that the new material remained stable on returning to room temperature and pressure and indeed superconducts at atmospheric pressures, but at just 3K.12
As well as making new materials, high-pressure studies have geological relevance. The Bayreuth scientists have investigated what happened to iron oxide at the high pressures it experiences in Earth’s mantle.13 ‘Above 60GPa and 2000K, Fe2 O3 and Fe3 O4 phases decompose, releasing oxygen and forming Fe5 O7 and Fe25 O32,’ Dubrovinskaia explains. The amount of oxygen produced due to Fe2 O3 decomposition alone can be as high as 8–10 times the mass of oxygen in the modern atmosphere. This oxygen should play an important and previously unappreciated role in geochemical processes.
Olga Shebanova from Diamond Light Source in Didcot, UK, has used high pressure to produce a ‘forbidden’ alloy, from rubidium and platinum. According to Miedema’s theory of alloy formation, that shouldn’t be possible, because the two metals have very different charge densities. But pressures above 12GPa force a rubidium electron to move to a different orbital. ‘The charge density increases dramatically, and then the conditions are there for them to alloy,’ Shebanova says. The alloy then remained stable on returning to atmospheric pressure.
Moderately high pressures like this are currently more interesting for Shebanova than the far extremes, as they might more readily be practically exploited. ‘Pressures below 25GPa are already achieved by manufacturers using multi-anvil presses,’ she says. Such presses allow production in cubic centimetre volumes or larger, but unlike portable palm-sized DACs, multi-anvil presses are the size of a small room and weigh several tonnes.
Forced into use
One potential application that Shebanova has been working on is in the production of nanoparticle materials. High pressure is believed to be a driving force for self-assembly, with 3D networks formed below 15GPa, Shebanova explains. At Diamond she has been studying the way in which nanoparticles assemble using extreme high pressure small angle x-ray scattering (EHP-SAXS), with SAXS especially well-suited to studying nanoscale structures. ‘The distances between particles can be tuned by varying pressure and other conditions, and that varies optical and electronic properties,’ Shebanova says. ‘You can manipulate electromagnetic waves, absorb, block and bend them, which can be used in applications like optical filters and sensors.’
While it’s not clear that any unusual chemistry is involved in such assembly processes, high-pressure strangeness may play a part in recent work on biocompatible polymers. Producing biocompatible hydroxylethylmethacrylate (HEMA), used in contact lenses, normally requires reactive initiator molecules and high temperatures. ‘That compromises its biocompatibility,’ Shebanova says. Pilot studies by Andreas Zerr and Philippe Djemia from the University of Paris North in France found that HEMA polymerises at pressures up to 7GPa without initiator molecules. Shebanova is now using EHP-SAXS to help them see how the polymer structure that they obtained is different to the conventionally produced structure.
Hoffmann too is exploring moderately high-pressure polymerisation, which he calls ‘the most exciting thing I’m working on right now’. Bo Chen, who works in Hoffmann’s group, has been helping scientists from Pennsylvania State University, US, unravel the polymerisation mechanisms that make what they call ‘carbon nanothreads’. By pressurising benzene to 20GPa at room temperature the Penn State researchers, led by Thomas Fitzgibbons, John Badding and Vincent Crespi, made polymers stiffer and stronger than carbon nanotubes.14 Their strength arises because every carbon atom is forced from making two single bonds and one double bond in benzene to four single bonds.
‘It’s exciting because those polymers can be brought back to atmospheric pressure,’ Hoffmann says. ‘They’re made in tiny amounts so far, but the promise is that there is a new way of synthesis.’ Hoffmann is also enthusiastic about high pressure chemistry generally for another, more philosophical reason. ‘If we understand things at the extremes we know more about the normal situation,’ he says.
Oganov’s desire is to find a simple rule that doesn’t involve intense calculations but will describe high-pressure behaviour. ‘I believe there must be a rule, but I have been trying to find it for years and any successes only explain narrow classes of systems,’ he admits. ‘Either this rule will not be found, or it will be found with completely tangential thinking. It seems to be a rather hard problem.’
Andy Extance is a science writer based in Exeter, UK
References
1 Y Ma et al, Nature, 2009, 458, 182 (DOI: 10.1038/nature07786)
2 A R Oganov and C W Glass, J. Chem. Phys., 2006, 124, 244704 (DOI: 10.1063/1.2210932)
3 W Zhang et al, Science, 2013, 342, 1502 (DOI: 10.1126/science.1244989)
4 Q Zhu et al, Nat. Chem., 2013, 7, 61 (DOI: 10.1038/ nchem.1497)
5 M-S Miao et al, Acc. Chem. Res., 2014, 47, 1311 (DOI: 10.1021/ar4002922)
6 M I Eremets and I A Troyan, Nat. Mater., 2011, 10, 927 (DOI: 10.1038/nmat3175)
7 A P Drozdov et al, Nature, 2015, 525, 73 (DOI: 10.1038/nature14964)
8 M Einaga et al, Nat. Phys., 2016, DOI: 10.1038/nphys3760
9 L Dubrovinsky et al, Nat. Commun., 2012, 3, 1163 (DOI: 10.1038/ncomms2160)
10 L Dubrovinsky et al, Nature, 2015, 525, 226 (DOI: 10.1038/nature14681)
11 A N Kolmogorov et al, Phys. Rev. Lett., 2010, 105, 217003 (DOI: 10.1103/physrevlett.105.217003)
12 H Gou et al, Phys. Rev. Lett., 2013, 111, 157002 (DOI: 10.1103/physrevlett.111.157002)
13 E Bykova et al, Nat. Commun., 2016, DOI: 10.1038/ncomms10661
14 T C Fitzgibbons et al, Nat. Mater., 2015, 14, 927 (DOI: 10.1038/nmat4088)











No comments yet