Andy Extance discovers how new medical adhesives are overcoming the difficulties bodily fluids cause conventional polymers
A tiny premature baby girl is fighting for her life, held by hands covered in white latex gloves. Her body stretches from the heel of the underlying palm to the end of a finger, with her head supported on the wrist and legs dangling past the fingertips. Her mottled, drip-fed arm is the length of another finger, while her belly bulges distressingly and disproportionately, intricate intestines visibly pressing against her skin.
Surgeon Michael Harrison from the University of California, San Francisco, in the US uses the distressing picture to highlight the risks of the ‘foetal access’ techniques that he’s pioneered. Some conditions pose danger so great to unborn children that they force doctors like Harrison to operate while they’re still in the womb. Such surgery trades a greater threat for a lesser one. To do the operations, Harrison threads narrow instruments through small holes in the protective amniotic sac. However, this can potentially rupture the sac, leading to the baby’s premature birth and possibly its death.
‘It’s a terrible unsolved problem,’ Harrison says. He has therefore been developing protective adhesive materials that could be applied to the membrane before procedures to stop such ruptures. In doing so, he’s joined with US chemists like Robert Grubbs from the California Institute of Technology and Phillip Messersmith from the University of California, Berkeley. ‘If we can preseal the membrane, then you can put whatever you want through it and it won’t make a leak,’ he says.
If you’ve ever had a plaster on your finger fall off in the washing-up bowl you’ll know one reason why this is so difficult. Water is the nemesis of commonly used adhesive polymers. The small water molecules slip into networks of long polymer chains, and in between adhesives and the surfaces they’re joined to. Once there, water swells the adhesives, changing their properties, and begins breaking them down. And the amniotic sac, like many other places inside our bodies where adhesives could be useful, is full of watery bodily fluid.
Chemists are therefore developing systems that they hope will vanquish the liquid. Some are taking inspiration from nature, in the form of mussels and snails, while others have invented adhesives controlled by electricity. But for ultimate success they also have to prove their glues are effective, safe enough to go in our bodies, and useable in extremely close quarters.
Hunting for glues
Surgical adhesives and sealants already exist, primarily for stopping bleeding, and earned manufacturers around $1.7 billion (£1.2 billion) in 2015, according to research group Markets and Markets. 60% of that goes on biological adhesives like fibrin glue, using components isolated from blood plasma, with the enzyme thrombin breaking down fibrinogen to make a fibrin clot. While sticky, such clots do not form strong bonds, however, and break down within 48 hours. As the raw materials must come from human blood, their availability is also limited, and there can be concerns about disease transmission. Among synthetic adhesives, polymers similar to superglue, cyanoacrylates, have been used medically since at least the Vietnam war. However, they’re mostly used to glue together skin externally, as their breakdown releases toxic formaldehyde, and continued exposure to water weakens them.
A more innovative approach originated from David Mandley’s PhD research at the University of Loughborough, UK, in the early 1990s. He investigated materials whose adhesive properties developed in response to light, sponsored by Tissuemed, in Leeds, UK, where Mandley is now chief executive. The company realised that inside the body it would be only possible to light up small areas, so Tissuemed therefore dropped the light-curing aspect, and adapted the chemistry to enable larger products. The result, known as TissuePatch, has been sold since 2007 for sealing air leaks in the lungs amd the dural membrane enveloping the brain and spine after neurosurgery.
‘We often call TissuePatch “surgical cling film”,’ Mandley explains. Its top surface is a non-adhesive barrier layer of poly(lactide-co-glycolide) (PLGA), a widely-used polymer in medical devices. The lower surface is the company’s TissueBond adhesive, a copolymer of vinyl pyrrolidone (PVP) and acrylic acid (PAA), the latter partly functionalised with N -hydroxysuccinimide (NHS). ‘PVP and PAA provide initial tack, and then NHS reacts with proteinaceous tissue surfaces to form covalent amide bonds that hold the device in place longer term,’ Mandley says. After implantation, water and enzymes in the patient’s body degrade the patch, with PLGA disappearing within 12 weeks. The TissueBond layer lasts longer, but eventually also breaks down.
Mandley describes the areas TissuePatch operates in as ‘moist’ rather than ‘ultrawet’. ‘There are unmet needs in very wet situations,’ he explains. He emphasises that meeting such needs for products intended to go inside and degrade within people’s bodies brings challenges beyond the physical and chemical – they must also gain government approval. ‘It’s how these emerging products find their way through the regulatory process that could be challenging,’ Mandley says.
Cohera Medical, based in North Carolina in the US, has successfully navigated that process. It sells a polyurethane adhesive for which water is an ally, not a foe. Its isocyanate building blocks covalently bond with each other and nearby tissue in water’s presence, explains Cohera’s chief scientific officer Dottie Clower. Yet conventional polyurethanes based on toluene diisocyanate are unsuitable for medical use. ‘When that breaks down you’re producing toluene diamine, which is a carcinogen,’ says Clower.
Cohera was spun out of the University of Pittsburgh, US, in 2006 based on research done by Eric Beckman to find a safer alternative to existing polyurethanes. Beckman developed isocyanates that break down to form the amino acid lysine, already common in our bodies. The resulting adhesive, known as TissuGlu Surgical Adhesive, is ten times stronger than fibrin sealant, Clower says. Its first use is securing large flaps of tissue in cosmetic ‘tummy tuck’ surgery. Surgeons dispense TissuGlu from a gun-shaped applicator, with three parallel barrels delivering evenly spaced droplets. ‘TissuGlu is present throughout the healing period of about three weeks until the natural tissue has rebuilt its collagen bonds,’ Clower explains.
TissuGlu received CE Mark approval in Europe in 2011 and after a 130-person clinical trial was approved by the US Food and Drug Administration (FDA) in February 2015. Cohera has now ramped up for a limited sales launch in the US, according to Clower. Cohera also combines its medical polyurethane with silane chemistry to produce Sylys Surgical Sealant, enabling much faster curing than in TissuGlu without non-biocompatible catalysts. Sylys has been shown to reduce potentially deadly bowel leaks after surgery, for which it received CE Mark approval in 2014 and is currently being assessed by the FDA.
Mussel power
Whereas Cohera and Tissuemed’s products cover or close relatively large holes, Harrison does keyhole foetal surgery. He therefore needs tools far more intricate than large patches or applicators. ‘You have to put the adhesive in the tiniest potential space between the amnion membrane and the uterine wall,’ Harrison says.
His desire for precision led Harrison to collaborate with Messersmith, who emulated the byssus protein glues that anchor mussels to the seabed. Among these proteins’ key features is an unusually high content of dopa, an amino acid not directly encoded in genetic instructions. Dopa contains catechol groups that can attach to surfaces through various types of chemical bonding.
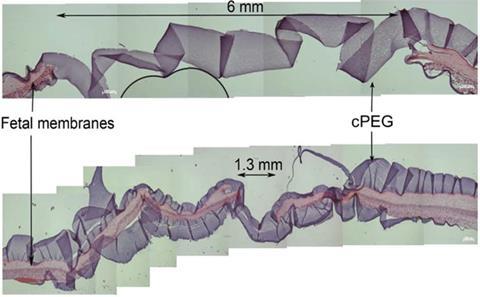
‘I visited San Francisco several years ago and did lab experiments with Mike,’ Messersmith recalls. ‘He and I, with our own hands, doing early stage stuff.’ Messersmith developed a polyethylene glycol (PEG) surgical sealant with high catechol group content suitable for wet environments. To make it gel, the polymer needs to be thoroughly mixed with sodium periodate prior to application in a short time period, which would pose problems in keyhole surgery.
‘Passing two solutions down a needle through several centimetres of tissue into a confined area where you don’t want the adhesive to spread brings significant engineering challenges,’ admits Messersmith. ‘But they’re not insurmountable.’ In tests in rabbits published in 2013, researchers in Belgium and Switzerland just pipetted the two parts separately, directly onto punctured foetal membranes.1 This crude approach increased foetal survival from 36% in control experiments to 80%.
Yet approval remains distant, with even the spin-out company Messersmith founded reluctant to pursue it. ‘In 2007, my company’s business guys looked into it and said “The market is just too small”,’ he says. ‘Investors want to fund ideas that are going to approach billion-dollar markets. So the commercial idea of adhesives for foetal surgery died.’ And in 2012 Messersmith’s original technology was acquired by the Netherlands-headquartered multinational DSM, which declined to comment on any aspect of its status.
Stuck for options
Harrison’s search for suitable pre-sealant adhesives subsequently grew to include Grubbs via Daniel Schwartz, a UCSF ophthalmologist they both knew. Guided by Schwartz, Hoyong Chung– a former member of Grubbs’ team – was developing a different two-part, mussel-inspired adhesive to remove chemical particles from patients’ eyes. One component of their adhesive is based on PAA, containing NHS like the TissueBond adhesive, but also dopa groups.2 The second component is based on PEG, and reacts when mixed with the first component to produce the final adhesive within 30 seconds.
We see a one-component system as the ideal
Philip Messersmith
The team has applied for patents based on this approach. Chung says that while it was just the ‘simplest and fastest way to show an initial possibility as a biomedical adhesive’, steps are being made to improve and commercialise it. Yet Chung, who is now continuing biomedical adhesive research in his own team at Florida State University, US, also emphasises that dopa doesn’t work ‘magically’. ‘The catechol group can only strengthen adhesion of a good polymer,’ he says.
Messersmith is applying this insight in developing a new adhesive generation. ‘We get very good adhesion, but when you apply force to the system it ruptures within the adhesive itself,’ he confesses. To overcome this, he again looked to mussels’ byssus protein networks, which are rich in iron, reinforcing the networks through non-covalent iron–catechol coordination. Messersmith’s team is now using iron crosslinks in its research.3 ‘They strengthen the adhesive,’ he says. ‘If you pull the adhesive, these sacrificial bonds rupture first, but they also reform when the load is removed.’ Messersmith is investigating simplified adhesive formulations that don’t need a separate periodate component. ‘I’m not giving up on a two-component system, but we do see a one-component system as the ideal,’ he says.
Snail-like progress
But others are already developing one-part adhesives for fluid-soaked minimally invasive operations, including Gecko Biomedical in France, whose founders include Massachusetts Institute of Technology biotechnologist Robert Langer. It too has taken inspiration from nature – but not geckos or even mussels, explains Maria Pereira, the company’s head of research. ‘Sandcastle worms and snails have remarkable viscous secretions that, even in challenging environments, remain in place,’ she says. ‘These secretions contain hydrophobic components that repel water.’
If we had something perfect today, it would barely be on the market within five years because of all the FDA clearances that you need
Terry Steele
Gecko Biomedical designed a blue-light-cured adhesive polymer made from glycerol and sebacic acid, two fatty monomers found naturally in our bodies.4 Its material is hydrophobic and viscous before curing, stopping it from being washed away by bodily fluids. ‘Its adhesive properties are only activated in a specific light wavelength, allowing the clinician to turn it on when correctly positioned,’ Pereira says. This tissue sealant, called GB02, is due to enter clinical trials in people in 2016.
Terry Steele at Nanyang Technological University (NTU) in Singapore is also researching light-cured tissue adhesives. However, he’s discovered another curing stimulus that he thinks is even better for hard-to-access areas: electricity. ‘Trying to get intense light into a fibre-optic and then exactly where your film is, that’s quite a high-tech engineering step,’ Steele highlights. ‘It’s going to be much easier to just put two copper wires in there.’
Electricity turns on unusual carbene crosslinking polymerisation chemistry. Carbenes are highly reactive radical species that rapidly assemble into polymer networks when they’re formed. Usually they’re made by exposing aryl–diazirine precursors to light, but Steele realised that carbenes had also previously been produced electrochemically. He also suspected this approach could be used in watery liquids like blood, although everybody he asked was sceptical, saying such reactions only work in organic solvents.
Undaunted, Steele asked his colleague Jianfeng Ping to try it. ‘He came back up and said “The experiments went all wrong: as soon as I applied voltage, it went hard”,’ Steele recalls. Misunderstanding Steele’s intention, Ping had been looking for a colour change. ‘He said, “I threw away the sample already!” and I said, “That sounds like something good – go get that sample!”’
The NTU team’s light- and electrically cured adhesives are intended as a way to fix drug-releasing films inside blood vessels.5 That could be useful, for example, to prevent scar tissue forming after angioplasty operations that seek to expand narrow arteries. Both adhesives are currently being tested in animals to check how tissue reacts to them. The lengthy approval process means that it will therefore be a long time before they’re used in practice – if they ever are. ‘If we had something perfect today, it would barely be on the market within five years because of all the FDA clearances that you need,’ laments Steele.
Yet a steady pipeline of new adhesive options is dribbling its way slowly onto the market, propelled by medical needs like Harrison’s, which Messersmith found ‘compelling’. ‘He’s such an inspirational person and so passionate,’ Messersmith says. ‘That made me resolve to get this properly resourced. I’m happy that finally we seem to be reaching that point. It may be years before we report anything significant, but at least we have this framework, and that makes it a lot easier.’
Andy Extance is a science writer based in Exeter, UK
References
1 A Kivelio et al, Eur. J. Obstet. Gynaecol. Reprod. Biol., 2013, 171, 140 (DOI: 10.1016/j.ejogrb.2013.09.003)
2 H Chung and R H Grubbs, Macromolecules, 2012, 45, 9666 (DOI: 10.1021/ma3017986)
3 D G Barrett et al, Adv. Funct. Mater., 2013, 23, 1111 (DOI: 10.1002/adfm.201201922)
4 N Lang et al, Sci. Transl. Med., 2014, 6, 218ra6 (DOI: 10.1126/scitranslmed.3006557)
5 J Ping et al,Nat. Commun., 2015, 6, 8050 (DOI: 10.1038/ncomms9050)









No comments yet