
‘You can consider every chemical reaction as a movement of charge from one place to another…it’s always electrons moving,’ says Stefan Matile, whose work with UK chemists has provided backing for a two-decades-old theory that an oriented external electric field can accelerate and direct electrons during a molecular transformation. The research offers a simple, environmentally friendly and cheap method for multistep synthesis with a high level of external control.
The researchers from Switzerland and the UK collaborated after observing that the reactor used by Thomas Wirth at the University of Cardiff, which had industrial success in radical chemistry, could be applied to Matile’s work on anion-π electrocatalysis at the University of Geneva. ‘It’s much more interesting what we can see before the electron jumps from the electrode into the reaction – we just use the [electric] field directing the electrons that are already there,’ says Matile, adding that they are interested in supramolecular interactions not redox chemistry.
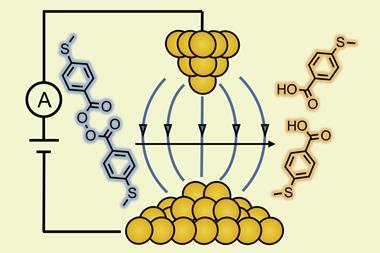
The reactor consists of two 5cm metal electrodes, coated in carbon nanotubes. The polarisability and conductivity of the carbon nanotubes are important – they produce a strong dipole that stabilises the ionic transition state of the reaction and amplifies the applied electric field. The plates are only separated by a 250μm thin fluorinated ethylene propylene foil that is printed with a flow channel. ‘The solution is always exposed to the field; the field is voltage divided by distance. The distance is small, so it is important to have a soft voltage,’ explains Matile. The short distance is also critical in avoiding unwanted electron transfer and the need for electrolytes. The substrate is injected into one side where it flows through the reactor, at which point a voltage is applied – flipping a switch – and a characterisable product is obtained on the other side.
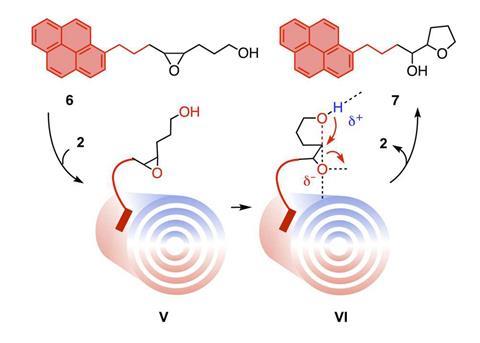
The researchers tested the reactor with an epoxide-opening ether cyclisation. Only once the electric field is applied, does the epoxide open and the negatively charged oxygen leave – activated by the carbon nanotubes. They induce powerful anion–π interactions, stabilising the electron rich transition state and, ultimately, accelerate the reaction rate catalysis.
Sason Shaik, a researcher at the Hebrew University of Jerusalem, Israel, says that the researchers improved on previous work by ‘adding new features, which makes the reaction proceed in a continuous flow system and generates quantitative amounts of the products’. Matile’s team measured the product conversion as a function of the applied field and found a quasi-linear relationship – the greater the electric field, the more product produced.
Shaik comments that the new research might lead to new materials and mechanistic discoveries. ‘The use of carbon nanotubes, which are delocalised and highly polarisable, gives rise to an ingenious method of catalysis based on electric fields,’ he adds.
Matile says that now that the proof of principle has been demonstrated, they are already working on expanding the pool of substrates adding that ‘it will be very easy to transfer [the system] to industry so it’s very promising’.
References
MÁ Gutiérrez López et al, Sci. Adv., 2023, 9, eadj5502 (DOI: 10.1126/sciadv.adj5502)















No comments yet