For the first time, researchers have mapped how collagen protein structures, which are structurally unstable at body temperature, unfold and refold in the body.
The researchers say that understanding more about the stability of collagen could aid research into connective tissue disorders associated with changes in collagen’s chemical compositions, such as Ehlers–Danlos syndrome and brittle bone disease.
Collagen is a threadlike protein made up of three intertwined chains of amino acids, twisted into a triple-helix structure. It is the most abundant protein in the human body, making up 15–30% of the total protein in our bodies.
In 2002, researchers showed that collagen proteins are not structurally stable at body temperature, meaning that if they do not manage to quickly join with other collagen proteins to form higher-order structures, such as fibrils, the helix unwinds and the chains fall apart.
Now, two researchers at Simon Fraser University in Burnaby, Canada, Nancy Forde and Alaa Al-Shaer, have used atomic force microscopy (AFM) to map which regions of the collagen sequence unravel first at body temperature and which regions are key to holding the chains together. They focused specifically on collagen IV, which is thought to be the first collagen to have arisen in evolution.
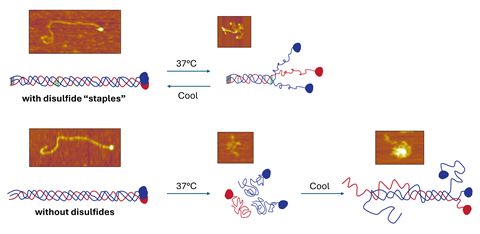
‘When you look at collagen IV, it’s got a globular end, the C-terminal for a peptide, and then a long chain, so it’s really easy to see in our AFM images,’ explains Forde.
Al-Shaer and Forde captured hundreds of AFM images at different temperatures of both intact collagen proteins and of collagen proteins that had unravelled. This enabled them to identify key molecular features that help the collagen to resist falling apart.

‘What Alaa found in our study was this particular type of collagen has disulfide bonds that keep the three chains together and provides a nucleation point … so they can zipper up,’ says Forde. ‘If you don’t have that, the chains can’t find each other to refold properly.’
‘We know eventually it will fall apart at body temperature, but if it can hold itself together for long enough that it gets properly secreted and then incorporated into the higher order structures … it’s much more stable,’ she adds.
Al-Shaer and Forde also cooled proteins that had unravelled and then observed them refolding. Forde says that the pair were surprised to find the collagen was able to refold in the opposite direction along the chain than had previously been thought possible. ‘People have applied electron microscopy imaging to study the folding, or unfolding of collagens, and they always interpreted their results as going from the C- to N-terminal direction. With this type IV collagen the cysteines are on the N-terminus … so it’s able to nucleate from this end and fold towards the C-terminus,’ she explains. ’This … had not been shown before.’
Unique insights
According to Forde, the work ‘sets the stage’ for learning how mutations from disease might disrupt this process and cause collagen to fall apart prematurely.
Matthew Shoulders, a chemist at the Massachusetts Institute of Technology in the US, describes the work of Forde and Al-Shaer as ‘a creative and compelling demonstration of the power of AFM to yield unique insights into collagen structure and function’.
‘The technique clearly has great promise for further illuminating the many mysteries of the triple helix,’ he adds.
‘This study demonstrates localised micro-unfolding, as well as nucleation of refolding, of the triple helix in natural collagen protein,’ says Jing Xu, a biophysicist at the University of California, Merced, in the US. Xu notes that she too was surprised by the refolding direction uncovered in the study.
‘The mechanism underlying refolding is conserved, suggesting evolutionary benefit of the stability of collagen IV protein, and providing new clues to the refolding pathway of the unstable collagen protein,’ she adds.
‘Collagen folding is a complex puzzle with many pieces including hydroxyprolines, interchain salt bridges, cysteine knots, chaperones and non-collagenous domains,’ says Abhishek Jalan, a biomaterials researcher at the University of Bayreuth in Germany. ‘How, and if, these cooperatively or individually direct folding is not clear.’
Jalan describes the finding around refolding direction as ‘intruiging’, adding that it ‘raises many questions’. ‘For instance, it is unclear how NC1 domains – which readily form hexamers due to high self-association rates – could persist as monomer while attached to the three folding chains that likely eliminate diffusion barriers to multimerisation,’ he notes.
References
A Al-Shaer and N R Forde, Proc. Natl. Acad. Sci., 2025, 122, 20 (DOI: 10.1073/pnas.2420308122)

















No comments yet