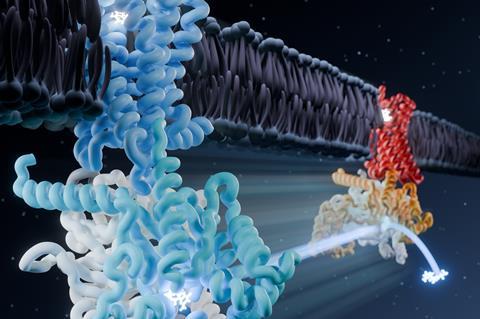
Cryo-electron microscopy has shed new light on the distinct steps by which opioids, and their antidotes, interact with the μ-opioid receptor.
It is hoped that these insights will provide a detailed roadmap of how these drugs work and aid the design of longer- or faster-acting antidotes or new opioids that have a reduced risk of addiction or side effects.
The μ-opioid receptor is one of the most clinically important of the superfamily of G protein-coupled receptors (GPCRs) but many drugs targeting the μ-opioid receptor are associated with a risk of side effects and addiction.
This has made the development of new drugs – both antidotes and new opioids – a priority. However, progress has been limited by an incomplete understanding of how the drugs work inside the cell.
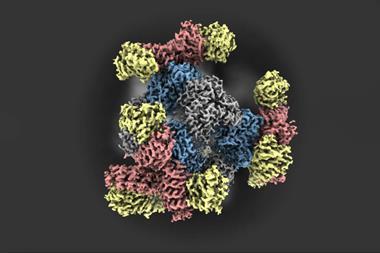
In this study, scientists based in the US used single particle cryo-EM to see how the μ-opioid receptor and its partner molecule – a heterotrimeric G protein that transmits signals inside the cell – change shape when an opioid or antidote binds. Signalling mediated by the μ-opioid receptor involves the transition of this G protein from an inactive form to an active state.
The researchers captured eight unique structural models and 16 cryogenic electron microscopy maps of the receptor with both naloxone – which reverses the effects of an opioid overdose – and loperamide – an opioid used to treat acute diarrhoea – capturing several intermediate conformations along the activation pathway.
The snapshots captured six different receptor states showing the initial steps of G protein engagement and activation – inactive, latent, engaged, unlatched, primed and nucleotide-free. They found that naloxone stalls the receptor in a ‘latent’ state, whereas loperamide promotes an ‘engaged’ state.
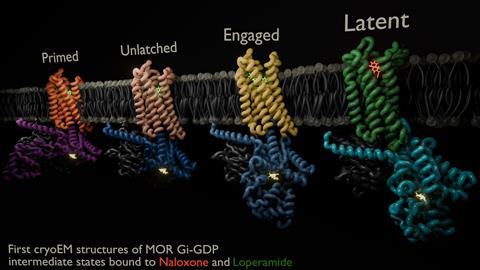
They also observed a direct correlation between the ligand binding pose and conformational states of the G protein; in the ‘inactive’ receptor conformation, naloxone was shown to adopt a shallow pose within the binding pocket, whereas in the ‘active’ conformation, both naloxone and loperamide engaged significantly deeper within the pocket.
The researchers said their findings also had applications beyond opioids: ‘Given the observation of functionally selective intermediate states giving rise to specific pharmacological outcomes, this model may prove more broadly applicable across the GPCR superfamily.’
References
S Khan et al, Nature, 2025, DOI: 10.1038/s41586-025-09677-6













No comments yet