By attempting to transform a substituted aldehyde into a substituted benzene, researchers have found a new and unexpected way to synthesise spiro[2,4]heptadienes.
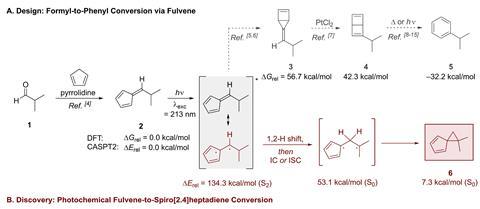
Computational studies, from the group of 2021 chemistry Nobel prize winner Ben List, calculated the reaction pathway to forming the substituted benzene. It was only when the experiment was undertaken in the lab that the unexpected bicyclic molecule spiro[2,4]heptadiene was formed, prompting the researchers to take another look at the intriguing result.
The unusual photorearrangement takes place after an aldehyde is reacted with pyrrolidine in a Knoevenagel-type reaction producing a fulvene. It was expected computationally that when treated with light the fulvene would photochemically rearrange to a bicyclic intermediate, containing both a three- and four-membered ring. This hypothetical intermediate, upon being treated with light and a platinum(II) chloride catalyst would undergo photoisomerisation, resulting in a substituted Dewar benzene and finally a substituted benzene. The group conducted DFT calculations to confirm this pathway, but when taking the reaction from the computer to the lab they discovered something strange.
The fulvene had rearranged itself upon irradiation, just not into the three- and four-membered ring bicyclic structure the DFT had predicted. Instead, the fulvene rearranged itself when treated by mercury UV lamps for 16 hours into a spiro[2,4]heptadiene. These compounds had previously been isolated due to their use as precursors to bidentate cyclopentadiene ligands, and chemists were intrigued by their unique structure. This method of synthesising them, however, was novel and the researchers were keen to understand how it happens.
Understanding the mechanism for the rearrangement was key. The group worked to replace the hydrogen atom in the alpha position to the exocyclic double bond in the fulvene with a deuterium atom. This simple change allowed the team to track the 1,2-hydrogen shift that takes place, forming the cyclopropane ring in the process. The team worked on synthesising substituted products starting with various substituted aldehydes, yielding six more examples of this rearrangement. With the discovery of this new synthesis route for spiro[2,4]heptadienes, the group will now turn their attention to the original hypothesis of converting the starting aldehyde into a benzene.
References
MM Lindner et al, Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202303119













No comments yet