Jamie Durrani tells the story of how two young upstarts, Ben List and David MacMillan, created a whole new field of catalysis
‘Somebody is pranking us. I bet you a thousand dollars it’s not real. I’m going back to bed.’
That was David MacMillan’s reply to a text message from Benjamin List saying the Royal Swedish Academy of Sciences had just called to tell him that the pair had won the Nobel prize in chemistry. But it wasn’t a prank. As MacMillan told the Nobel Foundation later that day, he’s ‘a thousand dollars down, but a very happy person!’

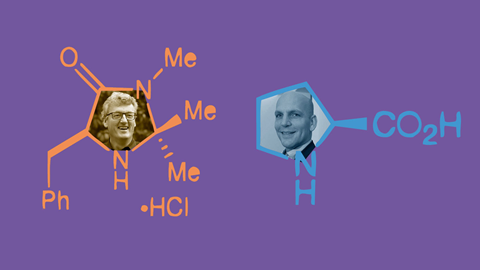
On Wednesday 6 October, List, a chemist from the Max Planck Institute in Mülheim and Princeton University’s MacMillan were awarded the Nobel prize ‘for the development of asymmetric organocatalysis’. Such has been the impact of their work, many people close to them had been expecting the call from Sweden to come for several years.
Often described as the ‘third pillar of asymmetric catalysis’, organocatalysis offers synthetic chemists an alternative way of making chiral molecules that does not rely on transition metals or enzymes.
#JustAnotherWednesday pic.twitter.com/Q35zhylsA8
— MacMillan Group (@MacMillan_Lab) October 6, 2021
The story of List and MacMillan’s achievement begins in the late 1990s in California, when both researchers started off their independent academic careers, List at the Scripps Research Institute in La Jolla and MacMillan at the University of California, Berkeley.
The early days
After finishing up a postdoctoral project at Harvard, MacMillan had grown tired of spending whole days at a glove box working with highly air- and moisture-sensitive transition metal catalysts. Arriving at Berkeley in 1998, his ‘big dream’ was to come up with a way to catalyse reactions using simple, small organic molecules ‘that everyone has in their stockrooms’. The plan was to design a molecule that could store and transfer electrons, in much the same way a metal centre accepts and donates charge during a traditional catalytic cycle.
‘I knew that this idea was definitely the way I wanted to go,’ says MacMillan. ‘I had no idea how to do it though, that was the problem!’ After some fruitless initial efforts, a lightbulb moment came when a graduate student – Tristan Lambert, now a professor at Cornell University in New York state, US – asked MacMillan a simple question about the mechanism for a reductive amination reaction.
As he explained that the key was in the formation of an iminium ion, which lowers the energy barrier for the reaction, MacMillan realised that this could be the exact phenomenon he’d been searching for. Below the mechanism he’d sketched out for Lambert, MacMillan drew up a scheme for reaction between α, β-unsaturated aldehydes and cyclopentadiene. ‘I thought this should work as an organocatalytic reaction,’ says MacMillan. ‘I was really excited about it.’
Building on older foundations
While MacMillan was scribbling on his whiteboard, List was planning the first experiments of his independent research career. After finishing a PhD on total synthesis at Goethe University Frankfurt in Germany, List had moved to Scripps to work with Richard Lerner and Carlos Barbas III on projects involving catalytic antibodies – enzymes that can promote all sorts of different chemical reactions.
During this postdoc, List worked with x-ray diffraction scientists to examine aldolase antibodies, looking for clues as to how they worked. Despite the low-resolution images available at the time, List explains that, looking closely, you could see that right in the enzyme’s active site were an amino group and an acid group that were crucial to its reactivity.
‘As my independent programme, I wanted to work with organic small molecules that had that machinery implemented into them, to catalyse aldol reactions and related chemistry. That was my plan,’ says List. ‘So I had this idea, and I sketched small molecules that have an amino group and an acid group.’
List knew of work from the late 1960s and 1970s on the Hajos–Parrish–Eder–Sauer–Weichert reaction, in which the amino acid proline catalyses an intramolecular aldol reaction. The process could be used in the synthesis of steroid molecules, but despite those early efforts, few attempts had been made over the intervening years to fully explore this chemistry.
‘The truth is this was well-known chemistry but somehow it had not been used on a technical scale,’ says List. ‘Even though it was discovered in industry, it never really became a process because there was an abundance of natural products with which they could make the steroids,’ he explains.
‘Somehow this chemistry fell asleep, in a way. It was used occasionally here and there and people had thought about the mechanism, but somehow they didn’t bring it together,’ he adds. ‘I don’t want to diminish anybody’s contribution here – it’s just for me, then, there was a realisation: maybe that is how proline works – it works like our catalytic antibody, you need an amino group and you need an acid group.’
It was just so beautiful it had to be true
Setting out to test the idea in the lab, List doubted whether something that seemed so simple could really have been overlooked for all these years. ‘When I did this first experiment, I was a bit nervous and I felt very insecure about it,’ he recalls. ‘I didn’t speak to many people about it, because I had this feeling that maybe it’s a naïve idea and all the other chemists – the smart chemists – already know there’s no way this is going to work.’
‘I remember doing the very first experiment, it was my very first independent experiment: the proline-catalysed aldol reaction of acetone with para-nitrobenzaldehyde.’
List hoped that the proline would bind to the acetone to form a reactive enamine intermediate, which would then drive forward a reaction with the aldehyde. With a bit of luck, the proline’s chirality could impart enantioselectivity on the reaction, by influencing the direction from which the aldehyde would approach.
‘In each step you need the acid or its conjugate base form, and it’s a really beautiful mechanism,’ says List. ‘It was just so beautiful it had to be true, I felt.’ If List’s theory was correct, the idea should be transferable to many more substrates and a plethora of different reactions.
‘I took a TLC the next morning, and I knew, wow, it’s working!’
A nervy process
As things were falling into place for List, MacMillan’s group were also seeing exciting results in the lab. After his realisation at the whiteboard, MacMillan asked one of his PhD students to investigate the idea of harnessing iminium ions in catalysis.
‘I was one of the first graduate students in the group – there was a group of seven of us,’ recalls Regis University chemist Kateri Ahrendt. ‘And so I was the first person in the group to start working on the asymmetric organocatalysis.’
‘Dave had proposed the project idea of using chiral amines to form iminium ions, which is reversible,’ she explains. ‘The chiral iminium ion would then accelerate the Diels–Alder reaction asymmetrically.’
I was just blown away that the thing even worked
MacMillan remembers Ahrendt seemed disappointed when she reported her first results to him. She’d used a proline methyl ester to catalyse the reaction but had only achieved an enantiomeric excess (ee) of 50% – meaning that while 75% of the product was formed as one enantiomer, the remaining 25% was formed in the opposite configuration.
‘I asked “Well, what do you mean – 50% ee, why don’t you think it worked?”’ says MacMillan. ‘And she was only a first-year graduate student at the time, and she said, “Well, aren’t we trying to get 90%?”’ he recalls.
‘I was just blown away that the thing even worked,’ says MacMillan. ‘She went back to her desk, I went into my office and started jumping up and down. I remember going home and telling my wife that night: “I think we just got tenure!”’
MacMillan, like List, realised the importance of seeing any reactivity at all. The catalyst could be modified to improve selectivity. As long as the iminium ion concept worked, they should be able to build upon it.
Ahrendt’s result brought a dilemma for MacMillan’s young team. Within 10 days, they had three reactions working, but the asymmetric control was still low – should they publish straight away to illustrate the concept, or continue fine-tuning the system to improve the enantioselectivity?
‘We actually spent another six months developing catalysts to get it into the 90% ee range,’ says MacMillan. ‘It was the most unbelievably nerve-racking six months of my life, waiting to see if we’d be scooped – if someone else would do this.’
It was a really, really fun moment because we’d been working so hard to get the right catalyst
The key to pushing up the selectivity was figuring out how to control the iminium ion geometry. ‘We made these funky C2 symmetric versions, which in some cases got the ees above 90%, but they weren’t really general,’ says MacMillan. ‘And then Kateri actually came up with a catalyst which was in the [eventual] paper.’
Ahrendt had been surveying a range of secondary amines, and realised that MacMillan’s C2 symmetric catalysts in particular were expensive and difficult to make. ‘I had this idea of the imidazolidinone, and so I was late at night, just under the radar, making those,’ she explains. ‘The first imidazolidinone catalyst was derived from phenylalanine. It has this phenyl ring, which blocks one face in the Diels–Alder reactions, so that’s how you get the enantioselectivity.’
Ahrendt got the first results with her imidazolidinone catalyst late one evening and took them to MacMillan straight away. ‘I remember her showing me the result, I think it was 93% ee and that was it, we just had a party that night,’ says MacMillan. ‘It was really great. It was a really, really fun moment because we’d been working so hard to get the right catalyst.’
‘It was incredibly exciting, it was probably one of the most fun projects I worked on – developing the catalysts for this method,’ says Ahrendt, who would later finish her PhD in another research group, opting to remain at Berkeley when MacMillan relocated his group to the California Institute of Technology. ‘I love data, I love coming up with new structures and then testing them and seeing the results and then interpreting what we can change about this scaffold to make it more reactive and more selective. So it was an incredibly fun, fun project.’
Prize and prejudice
List and MacMillan wrote up their findings and submitted manuscripts to the Journal of the American Chemical Society within one month of each other. The two articles, published in early 2000, were at the heart of the Nobel committee’s decision to award the pair the Nobel prize. Amazingly, these papers were just List’s first and MacMillan’s second as independent researchers.
But in those early days, the two didn’t always get the warmest of responses when presenting their findings. Many questioned whether this was really such a new innovation and if the concept could possibly be as translatable as MacMillan and List believed.
They really had to persevere in spite of those sometimes very unfriendly reactions from the community
‘There was a lot of dismissive reaction from the community,’ says Nuno Maulide, a chemist at the University of Vienna in Austria. He recalls attending talks by both List and MacMillan where audience members reacted derisively to the young upstarts claiming to have discovered a whole new field of catalysis.
‘I remember in the first years people would make fun of me after my talk, saying “I wouldn’t even call proline a catalyst – it’s a sub-stoichiometric reagent at best!”’ remarks List.
‘They really had to fight against this,’ says Maulide, who worked alongside List in Mülheim between 2009 and 2013. ‘They really had to persevere in spite of those sometimes very unfriendly reactions from a community, which (a) is very conservative and (b) looked at this and said “My God, this is so basic and so trivial and so simple, even an undergraduate student understands it. It cannot be that general, it cannot be that powerful…”’
Beyond their very first demonstrations of selective enamine and iminium ion catalysts, many people – including the Nobel prize committee – note that List and MacMillan’s major contribution was in ‘conceptualising’ the field of organocatalysis. While earlier researchers had sporadically worked with organic catalysts, these projects were rarely, if ever, followed up. List and MacMillan – alongside a few early entrants, including List’s former colleague Barbas who died at the tragically young age of 49 in 2014 – continued developing new catalysts, proving that organocatalysis was a broad idea that could be used in many reactions. As further papers were published showing organocatalysts being applied in a range of different scenarios, Maulide notes, ‘more and more people jumped on the bandwagon’.
Democratising chemistry
‘Basically, the two made this big statement: this is a general concept, which then meant that everybody could do this,’ says Manuel van Gemmeren, a former PhD student of List’s who now heads a research group at the University of Münster in Germany. ‘And everybody really meant everybody … it was not one of those things that can only be done at Ivy League universities in the US. It can be done at any lab that has access to absolutely basic chemicals.’
They showed that these reactions were really accessible: you don’t need a fancy glove box, you can readily buy amino acids for dirt cheap
It was a ‘democratising moment’, according to Maulide. ‘Our discipline typically is very elitist, in the sense that if you want to do certain things, you have to have a certain know-how and you have to have certain equipment, you have to have a certain environment and you need to have a certain training,’ he explains. ‘But here was a type of chemistry that anybody – as long as they had solvents, chemicals and stir bars, without even needing to dry things – everybody could just think “I want to try this.”’
‘It’s not just about pitching the chemistry, but it’s contextualising it in a way that proves to be more general,’ explains catalysis researcher Christine Le from York University in Ontario, Canada. ‘It’s not some esoteric reaction that’s applicable to maybe one class of natural products or whatever, it is a general methodology that can be broadly applied. And I think that’s where the power of their contributions made a difference.’
It was in MacMillan’s 2000 JACS paper that the term ‘organocatalysis’ first appeared – something he has previously said he believes helped to ‘ignite’ the field. ‘It’s really catchy when somebody coins a particular name for a type of catalysis, which both List and MacMillan are really good at doing,’ says Le. ‘And so the field just kind of catches on. And they showed that these reactions were really accessible: you don’t need a fancy glove box, you can readily buy amino acids for dirt cheap. And so I think that really led to the boom of this chemistry after their initial findings.’
Boom and bust ups
Within a decade of List and MacMillan’s first papers on the topic, more than 1000 papers a year were being published in the general area of organocatalysis. In the first years, List and MacMillan applied their chemistry to a wide range of reactions, including three-component reactions and catalytic cascades. Organocatalysis began to find use in all sorts of total synthesis projects and the field soon attracted interest of pharma companies keen to exploit new ways to construct complex bioactive molecules.
Many other notable chemists found new, creative ways to expand organocatalysis’ scope. ‘There are those people that pioneered the hydrogen-bond donor thiourea-type catalysts like [Harvard’s] Eric Jacobson and Peter Schreiner here in Germany,’ says van Gemmeren. He also lists Karl Anker Jørgensen, Paolo Melchiorre, Takahiko Akiyama, Masahiro Terada, Hisashi Yamamoto, Frank Glorius and Thorsten Bach among early innovators of the field who ‘used the general concept of organocatalysis and applied it to something completely different’. ‘There’s really a lot of people who introduced their own catalysts, their own modes of action and this was, I think, essential for the explosion of this field.’
The early days were tough and very competitive
As more chemists waded into the topic, competition to be the first to publish a new reaction or develop a better catalyst became fierce. The early years were pockmarked with disputes between researchers and even accusations of fraud. With MacMillan and List at the forefront of the field, an intense rivalry emerged between the two groups.
Maulide remembers helping to organise a conference that List was chairing at Mülheim in 2010. ‘It was a real who’s who of organocatalysis, everybody was there,’ he says. ‘We were all a little bit in trepidation, wondering: the rivalry between List and MacMillan – how is this going to turn out? They are both going to be there, and List’s actually the host – how is MacMillan going to behave?’
In the end, MacMillan was ‘extremely gracious’, says Maulide. ‘I was there at the dinner, where afterwards they were having drinks and beers … the two of them were really clearly and openly in front of everyone, burying whatever hatchet there might have been.’
‘The early days were tough and very competitive. With Dave, we had a few overlaps here and there,’ says List. ‘But fortunately, the two of us realised how silly these arguments were – about certain aspects of organocatalysis, like how to name it, or who was first on this or that – when the big picture is we created a new field that was super-exciting, that was about to change, maybe even revolutionise, asymmetric synthesis.’
Extending their legacy
Since their initial discoveries, MacMillan and List have continued to push the limits of asymmetric synthesis. Shortly after moving to Caltech, MacMillan and his students Joel Austin and Chris Borths developed a second generation of the imidazolidinone catalyst that was even broader in scope than Ahrendt’s first catalyst. Then in 2007, MacMillan introduced the idea of ‘Somo’ catalysis, which brought together organocatalysis and free radical chemistry to open up a new way to react electron-rich compounds that had previously been out of reach. A year after that, he turned his focus to photoredox catalysis, and has helped drive a spectacular expansion of the field. More recently he has shown how photocatalysis can be used to better understand the interactions of proteins on cell surfaces, with a powerful new technique he dubs ‘micromapping’.
‘Dave is always really focused on the big picture and trying to find new space,’ says Rob Knowles, a former PhD student of MacMillan’s and now colleague at Princeton. ‘It’s less about the sort of specific transformations or outcomes, and it’s really a little bit upstream of that – trying to think about new concepts and ideas that ideally will be impactful broadly,’ he says.
OK, this is a solved problem. Now, what’s the next big thing that needs to be tackled?
‘People can take those ideas and adapt them to all sorts of different purposes and create all sorts of downstream impacts,’ Knowles adds. ‘And so I really think that’s, in a nutshell, why he’s been and continues to be so successful.’
List meanwhile made enormous strides in the area of asymmetric counteranion directed catalysis (ACDC), where the enantioselectivity of a reaction is dictated by the structure of a chiral phosphate anion rather than the cationic intermediate itself. Recently, List’s group have shown that these reactions can work with staggeringly low catalyst loadings. ‘We have now catalysts that are the most active chiral catalysts that have ever been made for any carbon–carbon bond forming reaction, with sub-ppm level catalyst loading,’ he notes.
His group has also designed enzyme-like catalysts with complex structures and confined active sites that exercise evermore intricate levels of substrate control, and has even created ‘organotextile catalysis’ – immobilising organocatalysts onto nylon, as a way to demonstrate how they could be contained, separated and recycled in industrial contexts.
‘I think something that Ben has always done is to think directly: “OK, this is a solved problem. Now, what’s the next big thing that needs to be tackled?”’ says van Gemmeren. ‘He’s not a big fan of follow-up papers to get the maximum out of one single achievement. He would really sometimes say “OK, but this is just a follow-up, let other people do follow-up chemistry. We have to find the next big challenge.”’
Making an impact
Despite the huge innovation seen with asymmetric organocatalysis, some critics argue that it hasn’t yet had the full impact that was often predicted. This could simply be because of the slow rate at which industry adopts new manufacturing strategies. However, List points out that one antiviral drug used to treat HIV is made using organocatalysis, while MacMillan notes that the Swiss fragrance company Firmenich uses organocatalysts in its production of a compound known as the ‘bloom constituent’.
‘There’s so many organocatalytic reactions and drug molecules that have been scaled up and went to pilot plants, and for different reasons – the molecules not making the clinic – it hasn’t made it completely to the world manufacturing scale,’ says MacMillan. ‘But there’s so many of them and those process groups have done amazing jobs of being able to figure out how to push those catalyst turnover numbers.’
Perhaps the biggest contribution of organocatalysis has been in drug discovery. Many researchers in academia and pharma companies use organocatalysis routinely when designing new molecules to test for biological activity. A chiral scaffold built quickly with an organocatalyst can then simply be decorated with other functional groups to provide a diverse set of molecules to screen.
I sometimes think that a Nobel prize is almost too much for a couple of people
Stuart Conway, a synthetic chemist based at the University of Oxford in the UK, believes the clearest evidence of organocatalysis’ impact can be found in every chemistry department, including in his own lab. ‘We are interested in making biologically active molecules to study biology, but most of what we do is make molecules,’ he explains. ‘We use organic chemistry every day. And there are now processes and reactions that we do that involve organocatalysis. And it’s just normal to use it.’
Conway points out that other Nobel prize-winning techniques like ring-closing metathesis and palladium cross-coupling reactions are also examples of ubiquitous tools that allowed chemists to reliably do something that they couldn’t previously do. ‘We’ve used some of MacMillan’s chemistry in the synthesis of sugars and we’re currently using some organocatalytic reactions in the development of some biologically active lipid molecules,’ says Conway. ‘And again, we just came to this not particularly thinking “We want to use organocatalysis”, we just went for the best way to do it – and this is what was in the literature. So it’s had an extremely broad impact.’
Van Gemmeren notes that if you were to search for papers on ‘organocatalysis’ in a scholarly database (see graph above), the results might suggest that the field’s growth has begun to slow down. ‘But it hasn’t slowed because organocatalysis has become less important,’ he says. ‘But because the field has been become so diverse, very often papers containing organocatalysis use more specialised keywords to describe what they’re doing.’
In other words, organocatalysis isn’t the selling point any more. That these catalysts are now so accepted and widely used illustrates just how far the field has come in a little over two decades. List reflects that it is really all the work from students, colleagues and rivals across the globe who took the idea and built upon it that have convinced the Nobel committee to award this year’s prize.
‘I sometimes think that a Nobel prize is almost too much for a couple of people. Because in reality it’s not only my lab, but the whole field – all these people who have really worked hard and very competitively and very aggressively,’ he says. ‘[The Nobel prize] is an honour also for the field. And everybody who had contributed to this in the last 20 years should also feel proud and feel to have contributed. It was not just Dave and me.’
Jamie Durrani is a science correspondent for Chemistry World














1 Reader's comment