Low concentrations of hydrogen trapped in confined environments can unexpectedly burn steadily, sometimes forming intricate fractal patterns as they do. Researchers in Spain and Germany have recorded beautiful images and videos of the process, which hold important safety lessons for the increasing use of hydrogen as an energy source.
Mario Sánchez-Sanz from University Carlos III of Madrid (UC3M), Spain, warns that hydrogen leaks into narrow gaps, for example between hydrogen fuel cells and a surrounding protective case. In such environments, he and his colleagues show that hydrogen can burn as hard-to-see, low-temperature flames that are generally difficult to detect. ‘The possibility of having these flames near a fuel storage depot clearly increases the risk of an accident,’ he says.
Previously, researchers had thought that hydrogen couldn’t burn for long in narrow spaces, which have a large surface area-to-volume ratio. ‘One typically thinks that heat losses at the walls would extinguish the reaction,’ says UC3M team member Fernando Veiga-López. However, calculations performed with scientists from the Polytechnic University of Madrid (UPM) and prior experiments suggested that this wasn’t the case.
Veiga-López visited Mike Kuznetsov’s lab at Karlsruhe Institute of Technology, Germany, in late 2018 and early 2019 to investigate in more detail. As hydrogen flames are usually too dim to record, the team used precise Schlieren imaging techniques, capturing events at high speed. Hydrogen concentrations were also carefully controlled to reproduce calculated conditions. ‘It was a complicated technical search for something that we didn’t know was going to show up,’ Kuznetsov notes.
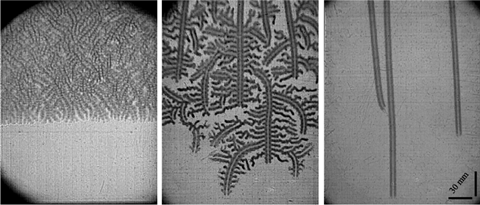
The experiments captured hydrogen burning in air as it travelled between two transparent sheets in three different ways. When the gap between the sheets was 3mm wide, the flame spread as a continuous solid front. At 2mm, the continuous front ‘breaks into a set of small flame cells separated by cold, unburned gas’, the researchers write. At a concentration of 10.5% hydrogen in air, this creates fractal-like flame propagation that ‘resembles the pathway of starving fungi or bacteria’. Reducing hydrogen concentration to 10.25% made the cells travel steadily in straight lines. Eventually reducing the gap width or hydrogen concentration did prevent the gas burning.
Edward Richardson from the University of Southampton says that the study is ‘very clear’. ‘It provides a beautifully illuminating presentation’ of combustion of dilute hydrogen mixtures, he says. The ‘diversity of flame structures that arise for these very weak hydrogen mixtures’ is notable, he adds.
References
F Veiga-López et al, Phys. Rev. Lett., 2020, DOI: 10.1103/PhysRevLett.124.174501



























No comments yet