Scientists have captured the first-ever glimpse of a stable germa-isonitrile bond. For more than 160 years, isonitriles (R–N≡C) have been valued as robust, versatile building blocks. Attempts at making their heavier group-14 analogues, known as tetrela-isonitriles (R–N≡E, where E is Si, Ge, Sn, Pb), have long fallen short.
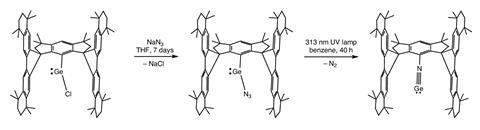
These N≡E triple bonds have existed only as fleeting, highly reactive intermediates, spotted indirectly in cryogenic or gas-phase studies, but never isolated in bulk or pinned down in a crystal structure. This is because heavier elements make the bond more polar and weaken π-bonding, causing molecules to stick together – instead of clean single molecules, chemists usually get clusters of dimers, trimers, cubic tetramers or even double-cubane structures, depending on the ligands.
Now, a research team from China has achieved something that had long remained an experimental challenge. They describe the first fully characterised, stable and bottleable germa-isonitrile (Ar–N≡Ge), kept intact by an exceptionally bulky aryl ligand.
Their strategy draws on earlier work showing that very crowded hydrindacene-based ligands can stabilise a surprising array of reactive, low-valent main-group species. The team reasoned that the same steric shield might slow the unwanted oligomerisation that usually plagues tetrela-isonitriles, giving a rare chance to isolate a stable monomer.
X-ray crystallography confirmed that the molecule features a true N≡Ge triple bond at room temperature, held in place by its exceptionally bulky ligands. Solid-state NMR and computational studies supported this picture, showing an unusually short and highly polarised Ge–N bond 1.64Å long. This polarity appears to underpin its unexpected reactivity: the team found that the molecule readily engages with organic electrophiles and transition-metal complexes, opening the door to chemistry that had previously been out of reach.
References
Z Wang et al, Nat. Chem., 2025, DOI: 10.1038/s41557-025-01997-4












No comments yet