Crystals of calcium bicarbonate have finally been synthesised, nearly 200 years after the mineral was first proposed to exist. The researchers say that obtaining and resolving the structure of such crystals (see video) ‘addresses a historical gap in both textbooks and contemporary research’.
Calcium bicarbonate (Ca(HCO3)2) is a well–known, water–soluble mineral. However, previous attempts to isolate crystals of the mineral from solution have failed, owing to the mineral’s tendency to decompose into more stable calcium carbonate (CaCO3) upon evaporation of water.
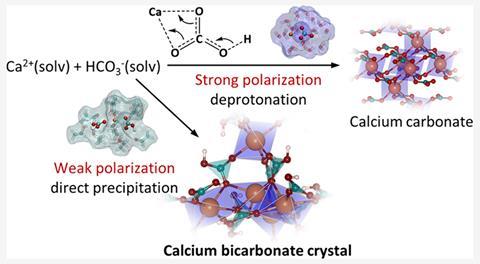
Researchers in China have now prepared the first crystals of solid calcium bicarbonate by using ethanol, a less-polar solvent, that helps stabilise the bicarbonate ions. The team pumped carbon dioxide into an anhydrous ethanol solution that contained dissolved calcium dichloride (CaCl2) and ammonia. This formed the required bicarbonate ions, which subsequently coordinated with calcium to form precipitates of calcium bicarbonate. Using the same strategy, the researchers also made bicarbonate crystals of strontium and barium, which were previously difficult to synthesise.
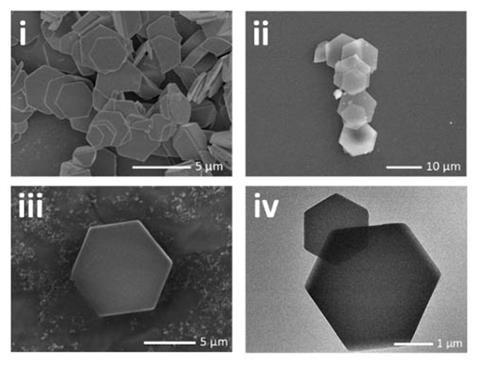
Diffraction experiments with calcium bicarbonate revealed a similar rhombohedral crystal structure to calcium carbonate. However, the new mineral has an increased porosity owing to the different binding modes of bicarbonate in the crystal, one of which helps bridge denser ionic layers. The uncoordinated hydroxy group may also further increase the distance between layers, with the researchers comparing it with the ‘dangling’ methyl group found in calcium acetate (Ca(CH3COO)2).
Computational analysis revealed that the decreased polarity of ethanol increases the stability of the O–H bond in the bicarbonate ions, preventing deprotonation and decomposition to calcium carbonate.
The researchers note that forming calcium bicarbonate crystals expands understanding of how metal–bicarbonate bonds form within ionic compounds. Materials such as these may also offer new ways to remove carbon dioxide from the atmosphere through direct mineralisation, they note.
References
K Kong et al, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c12101















1 Reader's comment