Infinite 3D polymers with precision porosity have opened up new horizons for chemistry. Here’s why this year’s Nobel prize looks at inner space
The 2025 Nobel prize in chemistry has been awarded to Susumu Kitagawa, at Kyoto University in Japan, Richard Robson, at the University of Melbourne in Australia, and Omar Yaghi, at the University of California, Berkeley in the US for their work on metal–organic frameworks (often called MOFs).
But what is a MOF? And what did the winners actually do? Here are the answers to some of the key questions about this year’s prize.
What is a metal–organic framework?
Metal–organic frameworks are materials in which metal ions are linked together by organic molecules in a regular repeating pattern to create a 3D network. Importantly, in the space between the metal nodes and the molecules that link them, there are large cavities that make the materials highly porous – speaking to Chemistry World back in 2017, Yaghi explained that just one gram of a MOF could have an internal surface area roughly equal to two American football fields.
This makes MOFs even more absorbent than other porous materials like zeolites and mesoporous silica. And it’s this feature that gives rise to the majority of MOFs’ most important applications.
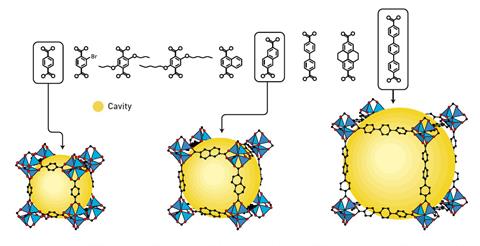
By using different metals and changing the type and length of the organic linkers, MOFs’ structures can be tuned to make the pores bigger or smaller, or to incorporate different functional groups, that will make the MOF better at binding specific guest molecules. This means that they can be optimised for use in things like gas storage, or various forms of filtration, or in carbon capture. The pores can also be designed to promote certain types of chemistry, meaning that MOFs have also found uses in catalysis, electrochemistry and fluorescence-based imaging.
Why are MOFs worthy of a Nobel prize?
During the Nobel announcement, the chair of the Nobel Committee for Chemistry, Heiner Linke, described MOFs’ ‘enormous potential’ that could unlock ‘previously unforeseen opportunities for custom-made materials with new functions’.
Robson’s first MOF was synthesised in 1989 (though he called them ‘coordination polymers’ or ‘infinite polymeric frameworks’), while Yaghi and Kitagawa’s work in the following years helped to define and develop the core concept. Since then, the field has exploded – in the early 2000s, just a few dozen research papers on MOFs were published each year; last year there were almost 10,000. And thousands of new MOF structures are reported each year, with over 100,000 reported to date. With this intensive research effort, numerous companies have been established seeking to commercialise MOFs for many different uses.
From a fundamental point of view, MOFs also represented a conceptual shift in terms of the way chemists design new materials. Rather than just tinkering with individual groups on a molecule, or linking them linearly to form polymers, MOFs offer the ability to controllably create a much larger extended and open 3D structure to optimise it for particular functions.
What did the laureates do?
In the 1980s, Robson decided to try to create a larger, synthetic variant of diamond’s famous network structure. Instead of tetrahedrally linked carbon atoms, Robson would use copper ions and an organic molecule with four ‘arms’. In an interview in 2019, he recalled that, at the time, most chemists would have thought that this mixture would have produced a ‘tangled bird’s nest’.
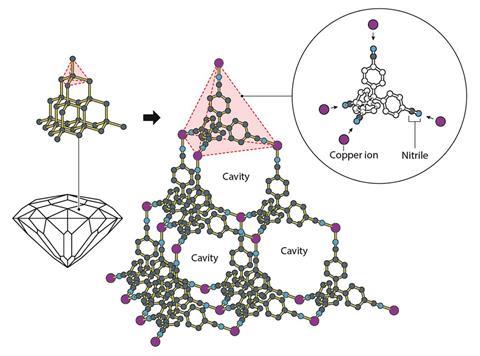
But Robson’s experiment worked, producing a regular, repeating crystalline structure – just as he’d predicted. When Robson and his collaborator Bernard Hoskins published their findings they described the material as ‘the first example of a deliberately designed and constructed infinite framework’.
Robson realised that that many, many more variants could be made using this design principle.

In the years that followed, Yaghi and Kitagawa made key contributions that helped to define this new area of chemistry and explored how these materials could be of real practical use.
In 1995, Yaghi coined the term ‘metal–organic framework’ when reporting on a new MOF, based on cobalt and carboxylate linkers, that could selectively bind pyridine molecules. He showed that the material was highly stable and capable of adsorbing, releasing and recapturing the guest molecules over multiple cycles.
In 1999, Yaghi unveiled what is now one of the most famous materials in the field: MOF-5. The team estimated the internal surface area of this zinc-based MOF at an enormous 2900m2 per gram of material. Key studies after this showed how families of MOFs could be rationally designed to fine-tune the size of their pores. Yaghi called this approach ‘reticular chemistry’.
Kitagawa’s major breakthroughs also came during the 1990s. In 1997 he designed MOF materials based on cobalt, nickel and zinc, that were intersected by open channels. These materials could absorb and release gases like methane, nitrogen and oxygen, without losing their structure.
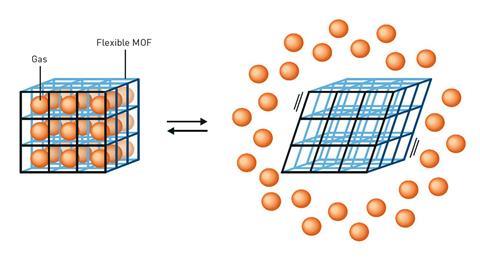
Kitagawa also developed flexible MOFs, dynamic materials that could change their structures and offer new functionality in response to pressure, temperature and light.
What are MOFs used for?
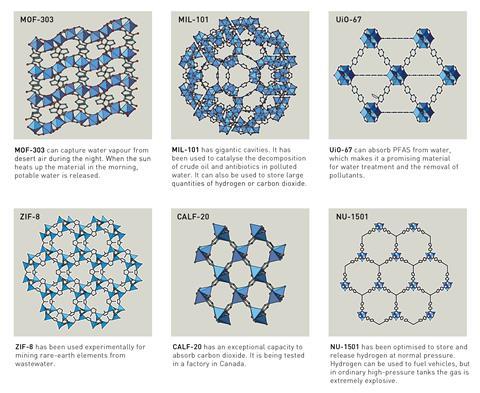
Applications for MOFs have become apparent over the last two decades. For example, Yaghi’s group has developed materials that selectively bind water molecules, enabling them to capture moisture from the desert air. Their ability to bind small gas molecules has also seen MOFs researched heavily for use in gas storage and carbon dioxide capture. Other groups have sought to use MOFs to capture per- and polyfluoralkyl substances from contaminated water.
They’ve also inspired other, more esoteric, projects including these incredible origami MOFs and beautiful designs that resemble mosaics from the Alhambra Palace.
A number of companies are now commercialising MOFs. In 2016, a UK company called MOF Technologies, now called Nuada, unveiled a product designed to prolong the life of fruit and vegetables by storing and slowly releasing a compound that regulates plant growth. That same year, US start-up NuMat launched a line of gas cylinders that use MOFs to safely store toxic gases that are used in the electronics industry.
Perhaps one of the biggest success stories is the CALF-20 MOF, which has been commercialised by the Canadian firm Svante in its carbon capture technology. This MOF can strip carbon dioxide from the waste gases in flues of industrial facilities.
The US tech giant Meta recently trained an AI on 15,000 known MOF structures to try to develop new materials that could capture carbon even more efficiently. That’s an approach that Yaghi also believes will make it easier to find the best MOFs for different applications, having recently told Nature that ‘by using LLMs and AI tools, we can speed up discovery from years to weeks’.















No comments yet