Rhenium catalyst that responds to visible light can effectively reduce carbon dioxide
A team of scientists in China and France has made a rhenium-based catalyst that reduces carbon dioxide under visible light, providing a practical alternative to UV-sensitive catalysts.
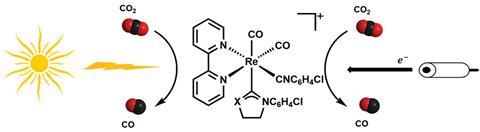
With atmospheric carbon dioxide concentrations increasing, scientists are searching for ways to halt the rise. One method is to capture the gas and convert it to chemical feedstock. But significant energy is required for its conversion. Considerable effort has, therefore, been devoted to developing catalysts for carbon dioxide’s electrochemical or photochemical reduction.
Rhenium carbonyl diimine complexes are one such candidate. The catalytic reduction process involves a metal to ligand charge transfer transition, where the movement of an electron from the metal to the ligand allows carbon dioxide to interact with the metal core. But UV light is needed to stimulate these transitions, and the catalysts perform poorly under visible light.
A group led by Chi-Chiu Ko at the City University of Hong Kong and Marc Robert at Paris Diderot University has now developed a set of rhenium based catalysts that respond to visible light. ‘We were thinking of a way we could modify the system such that a single component system is possible using visible light,’ says Ko. ‘The importance of this catalyst is that it could use visible light very effectively.’
To prepare these new catalysts the group built on their previous work in luminophores, atoms or functional groups responsible for a compound’s luminescent properties. Using ligand exchange to tune charge transfer transitions, Ko discovered that carbene ligands can improve a complex’s photocatalytic behaviour while also improving its stability. The rhenium complexes have excellent absorptivity and use low-energy visible light to catalyse reduction reactions. Robert’s group also studied the complex’s electrocatalytic properties and found they were highly efficient, performing the reduction under visible light.
‘We have a common interest, catalysing carbon dioxide to useful compounds, but our approach, molecular electrochemistry, is ideally complemented by the photochemical techniques and approaches developed by Ko,’ says Robert. ‘Collaboration is key to scientific success.’
Ding Ma, an expert in catalysis at Peking University, China, affirms visible light will be crucial in driving the field forward. ‘This work is a step forward to the organometallic complex approach,’ Ma tells Chemistry World. ‘The use of visible light is essential for the practical application of photocatalytic carbon dioxide fixing.’
The team now hope to develop metal complexes that can capture carbon dioxide, as well as reduce it.
References
C-C Ko et al, Dalton Trans., 2016, DOI: 10.1039/c6dt01686cC










No comments yet