New electrocatalysis electrodes have been created that are simpler and cheaper than conventional ones, and can substantially increase the efficiency of water splitting. Decorated with chiral molecules like helicenes, these devices double the activity of the oxygen evolution reaction, the bottleneck of the process, and improve its selectivity.
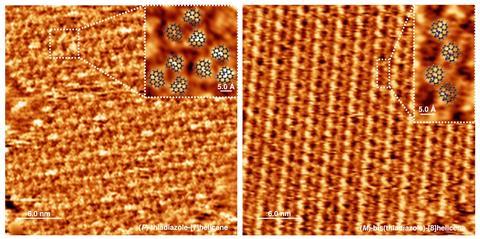
‘With electrocatalysis, we [can] use electrons from renewable sources [like solar and wind] to produce clean chemicals and fuels,’ explains Magalí Lingenfelder from the Max Planck–EPFL laboratory for molecular nanoscience and technology, in Switzerland, who led the study. In this work, her team focused on the oxygen evolution reaction. ‘It’s the bottleneck of water splitting,’ she says. ‘We wanted to increase its performance with cheap, simple solutions.’
They decided to investigate spin polarisation, an effect that had previously shown great promise boosting the activity of oxygen evolution. In molecules, electrons have either parallel or anti-parallel spins – filtering one spin orientation facilitates water splitting, because it favours the formation of oxygen. ‘In this case, chiral molecules filter the electrons with the same spin orientation – it’s an effect known as chiral-induced spin selectivity,’ explains Lingenfelder.
‘The ability to control electron spin at room temperature … will transform technology,’ explains Jess Wade, an expert in chiral materials at Imperial College London. ‘In this elegant study, [researchers] demonstrate that chiral molecules enhance spin-dependent catalytic reactions, like oxygen evolution.’ Wade notes that the enhanced selectivity of the process towards the formation of oxygen means fewer unwanted side-products like hydrogen peroxide. ‘Because hydrogen peroxide is a singlet species, [it] cannot form on a spin-polarised surface.’
The electrocatalysts feature a layered structure – a gold surface to anchor the chiral helicenes, then a layer of metal oxide to catalyse the water splitting reaction. Surprisingly, the upper layer is thin enough to preserve the polarising effect of helicenes. ‘The molecules enhance the reaction [without] being at the surface of the catalyst,’ explains Ifan Stephens, an expert in electrochemistry, also from Imperial.
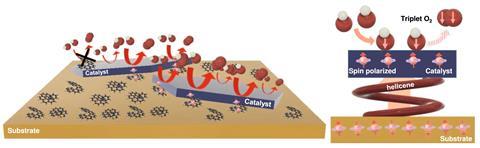
Lingenfelder claims this approach simplifies the reaction setup. ‘Before, researchers used magnetic fields to achieve similar spin polarisation, which requires special equipment around the flasks,’ she explains. Moreover, the team functionalised the same iron and nickel magnetic materials using their chiral molecules, and observed results ‘at least five times better’. The researchers also simplified fabrication. As the molecules self-assemble on the surface forming an ordered, layered structure, they could develop a spin-coating technique to build the electrodes. ‘It’s a standard technique, similar to the production of perovskite solar cells,’ she adds.
‘It’s a breakthrough [that] chiral molecules enhance the activity of nickel oxides [for the] oxygen evolution reaction,’ he adds. Normally, organic molecules would present great challenges in terms of stability. However, as molecules aren’t directly exposed to the alkaline electrolyte, long-term stability could become a reality, explains Stephens.
‘[This] provides an additional lever to tune oxygen evolution catalysts … it’s an excellent proof of concept,’ Stephens says. Additionally, it shows potential towards enhancing other spin-dependent electrocatalytic reactions. ‘[Helicenes] have huge versatility in terms of design and they’re cheap to manufacture,’ says Wade. This is in contrast with ferromagnetic materials, often more complex to control and operated under cryogenic cooling. Furthermore, ‘new chiral molecules [could] achieve high degrees of polarisation, [and] even more significant rates of enhancement’, she adds.
‘We used the oxygen evolution reaction to identify new design principles for spin selective electrodes,’ says Lingenfelder. ‘It’s amazing that chiral molecules control … reactions of non-chiral small molecules,’ she adds. ‘Surely our guidelines will open new possibilities to improve electrocatalysts in many reactions … such as the electroreduction of carbon dioxide.’
References
Y Liang et al, Nat. Commun., 2022, 13, 3356 (DOI: 10.1038/s41467-022-31096-8)














No comments yet