
The self assembly of molecules into supramolecular structures responds to a variety of triggers, including light, temperature, pH and chemical stimuli. Now, researchers have discovered that chemical fuels can drive molecules to self assemble into different structures – depending on these fuels’ handedness. This could provide an explanation for the origins of life’s homochirality – particularly in peptides and proteins.
‘Changing the chirality of [chemical] fuels creates different three-dimensional structures,’ explains senior author Charalampos Pappas, from the University of Freiburg in Germany. ‘This advances our ability to create synthetic systems with lifelike properties [and] could prove important for the origin of homochirality.’
The German team looked into acylation, a common reaction that ‘activates’ peptides, creating a chemical change that prompts processes like self assembly. However, when a chiral peptide reacts with a chiral acylating agent, the reaction yields two diastereomeric structures with different physicochemical properties. ‘[Such] supramolecular assemblies display strikingly different formation kinetics … as well as different structural morphologies and mechanical properties,’ says Jeanne Crassous, an expert in supramolecular chemistry and chirality at the CNRS Institute for Chemical Sciences in Rennes, France who wasn’t involved in this work. The researchers then characterised these supramolecular self assemblies using a comprehensive combination of techniques, including fluorescence and electron microscopy, to study morphology, and liquid chromatography–mass spectroscopy to measure the stability of the structures.
‘Using the left-handed acylating reagent creates an activated peptide with a different 3D structure to the one formed by the right-handed fuel,’ says Lenard Saile, who co-led the project with Kun Dai. ‘Imagine the peptide forms a ball when fuelled with the left-handed [reagent] and a sheet with the right-handed one,’ he adds. ‘While sheets stack easily, balls usually cannot and stay dispersed.’
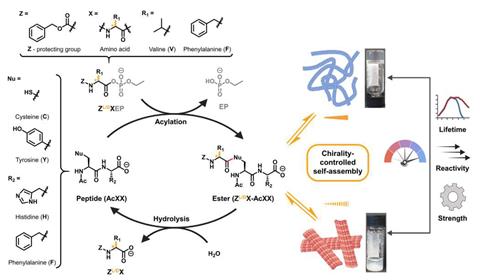
Similarly, the diastereomeric self assembled structures behave rather differently. In fact, their stability and resistance to hydrolysis also varies. This opens an opportunity to control reactivity and create ‘chemical computers’ and processes ‘tuned by subtle stereochemical information’, explains Saile. ‘In combination with other reactions and stimuli, chemists could create compartmentalised systems, [mimicking] metabolism and reproduction.’
Acylation reactions are fundamental biochemical construction tools , which control the behaviour of proteins in the cell. ‘For example, the acylation of histones regulates the expression of genes,’ says Saile. ‘Interestingly, most biological acylations involve an acyl phosphate as the activated acyl donor,’ he adds. ‘This is why we chose [chiral] acyl phosphates as biomimetics,’ he says – to better understand these systems. Such self-assembly processes, triggered by controlled chiral acylation, are artificial and ‘unused by natural systems’, according to Saile. Nevertheless, the discovery of a link between chirality and chemically controlled self assembly could uncover interesting hypotheses about homochirality in living systems.
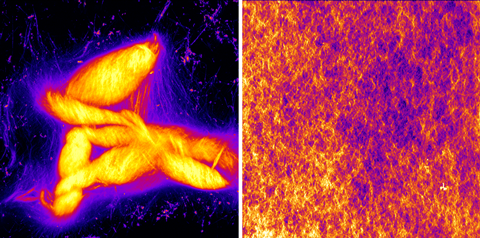
‘One handedness produces more robust and ordered assemblies than the other, which suggests chirality … could have dictated the nature of primitive supramolecular structures,’ explains Claudia Bonfio, an expert in the origins of life at the University of Cambridge, UK. ‘This shifts the question of chirality from single molecules to larger supramolecular assemblies.’
‘It is a process driven by the intrinsic identity of the building blocks, not an added component or a change in the environment,’ says Bonfio. ‘The paper raises the intriguing possibility that, on early Earth, mixtures of chiral peptides and acylating compounds could have biased self assembly, potentially provoking the divide in the different enantiomeric forms [of biomolecules].’
‘Chemically fuelled processes are a hallmark of life and make up nature’s marvellous ability to move and adapt,’ says Pappas. At the same time, a chemist’s toolbox expands by exploring mechanisms unprecedented in nature, like chirality-controlled acylation. Pappas now imagines creating multi-responsive, precisely controlled chemical networks – getting closer to the goal of creating living synthetic systems.
References
L Saile et al, Angew. Chem., Int. Ed., 2025, 64, e202508481 (DOI: 10.1002/anie.202508481)
















1 Reader's comment